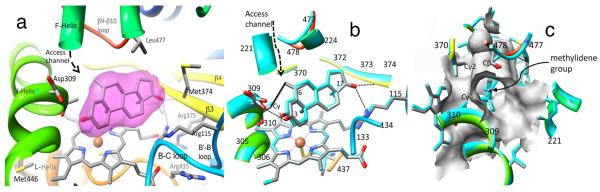
Figure 5.
Closeup views of the catalytic clefts of aromatase complexes. (a) The steroid-binding pocket of the bound EXM molecule and its unbiased (before inclusion of any small molecule or solvent in the model) difference (∣Fobs∣ – ∣Fcal∣) electron density at 4.5σ (PDB code 3S7S). The surrounding protein environment is shown and labeled. The protein backbone is rendered in rainbow color (N terminus, blue; C terminus, red): carbon, gray; nitrogen, blue; oxygen, red. (b) The superimposed catalytic clefts of the ASD and EXM complexes illustrating the deviation between two steroid-binding modes and the active site residues. The ASD complex is shown in blue backbone and blue carbon (PDB code 3S79). The complexes of the C6-substituted androgens 4/5 also have similar deviations. The van der Waals contact distance (3.4 Å) between the C6-methylidene carbon and Cγ of Thr310 is indicated by a black solid line. The hydrogen bonds between the exemestane and active site residues are drawn as dashed lines. (c) View of the catalytic cleft from the access channel perspective. A hydrophobic crevice formed by Thr310-Cγ, Val370-Cγ2, and Ser478-Cβ firmly grips the C6-methylidene group of exemestane. The van der Waals surface of the catalytic cleft for the aromatase–EXM complex is rendered in light gray.