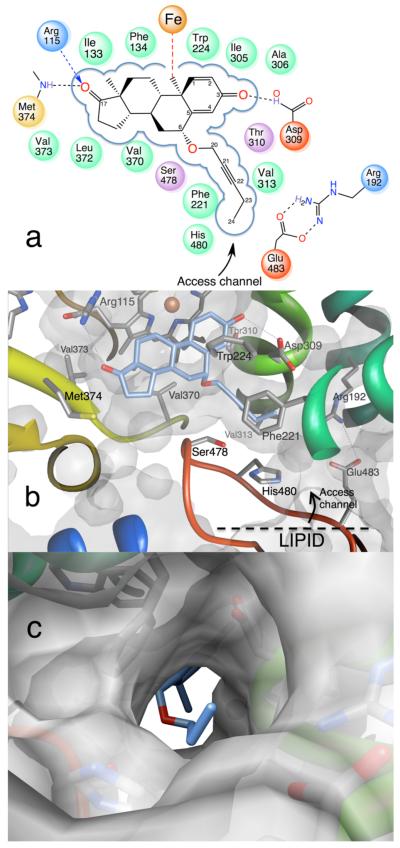
Figure 6.
Binding mode of the novel aromatase inhibitor C6β-2-alkynyloxy derivative 5. (a) Schematic diagram depicting the tight hydrophobic binding pocket for the steroid skeleton, the proton donors at the 3- and 17-keto positions, and the 6β-alkoxy-substituted alkyne side group that nearly fills the access channel. The color codes are the same as in previous figures. (b) View of the catalytic pocket and the access channel residues from the crystal structure of the aromatase-C6β-2-alkynyloxy derivative 5 complex (PDB code 4GL7). The bound molecule 5 (carbon atoms in light blue) is shown within the active site “pouch”, which results from the rendering of the van der Waals protein surface in semitransparent gray color. The access channel entrance at the lipid interface is the only opening of the “pouch” as indicated. See text for the details of interaction of the alkyne side group with access channel residues. (c) View along access channel entrance roughly normal to the viewing direction in (b).