Figure 5.
The Linker Is a Stable Domain when Undocked from the AAA+ Ring
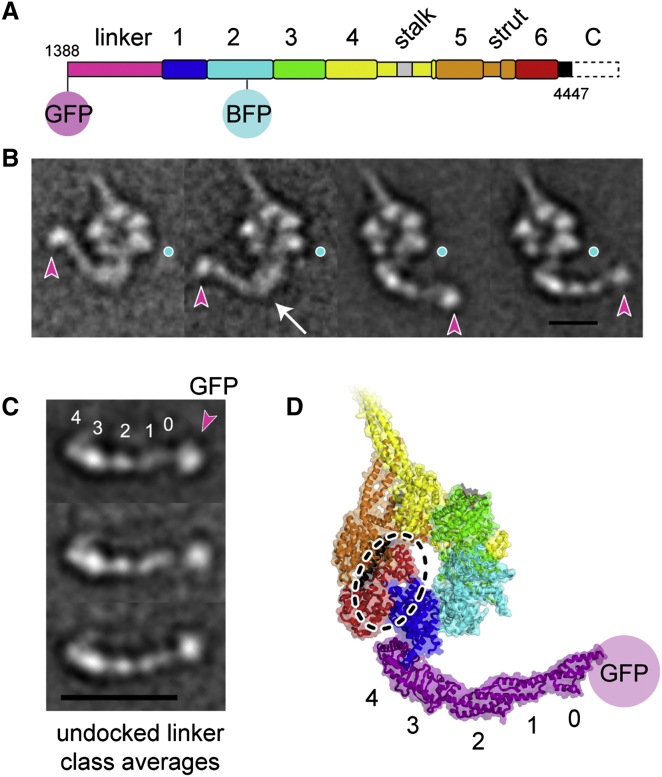
(A) Deletion of C-terminal amino acids 4,448–4,730 (dashed outline) from the cytoplasmic dynein motor yields a construct displaying frequent linker undocking in ADP. Analysis of the intact (i.e., linker docked) molecules is shown in Figure S3. Biochemical properties of this construct are described in Numata et al. (2011). The N-terminal tail domain is also deleted in this construct.
(B) Negative-stain class averages showing the linker undocked from the AAA+ ring. The GFP tag at the end of the linker is marked with a magenta arrowhead. The global mean position of the BFP tag in AAA2 is overlaid in each class (cyan disc). A sharp bend in the linker is only occasionally present when it lies to the left of the ring (white arrow). Scale bar is 10 nm. See also Movie S1.
(C) Class averages after alignment based on linker features showing that the linker is comparatively stiff along its length. The classes differ mainly in the position of GFP, which is flexibly attached to the linker. See also Movie S2. Five subdomains within the linker are numbered after Kon et al. (2012), and the GFP tag at the linker N terminus (GN) is indicated. Scale bar is 18 nm. See also Movie S2.
(D) Model of a linker-undocked molecule derived from D. discoideum dynein crystal structure PDB 3VKG (Kon et al., 2012). The precise part of the structure that deforms to enable undocking is not clear but might involve the C-terminal end of the linker or the N-terminal part of AAA1. The location of the truncated C-terminal region is shown with a dashed outline.