Figure 1.
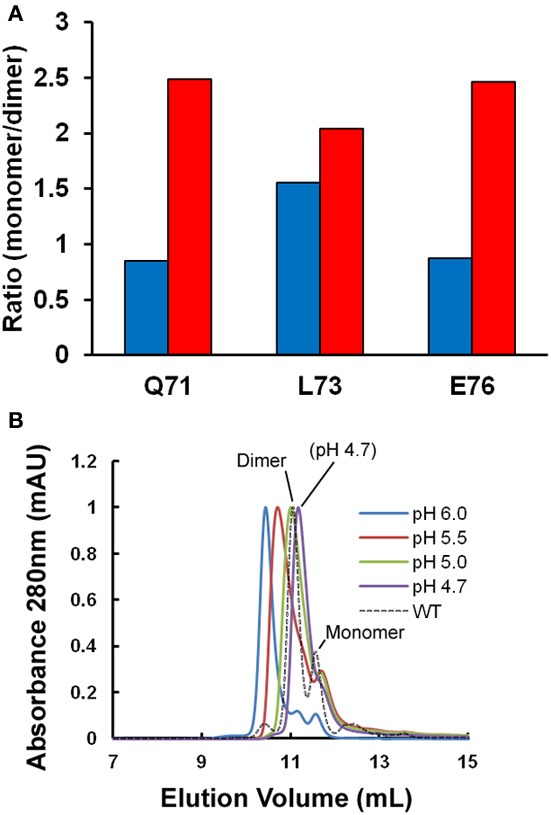
Oligomeric state of stefin B under fibrillization conditions in vitro. (A) Changes in the apparent ratio of monomer to dimer as the protein is taken from physiological pH (pH 6.0, in blue) to conditions which favor fibrillization in vitro (pH 4.7, in red). The ratio of monomer and dimer is calculated from amide peak intensities for three residues (Q71, L73, and E76) showing well-resolved NMR signal changes in the 2D 1H15N HSQC. Under the conditions of the experiment, we estimate the error to be ±0.25. (B) A single point mutation (P79S) causes the stabilization of the tetrameric state of stefin B in solution at pH 7.0 (Jenko Kokalj et al., 2007). Fibrillization conditions optimized in vitro cause dissociation of the tetramer to a mixture of dimer and monomer close to WT, which explains how this mutant remains a slow fibrillizing species.
