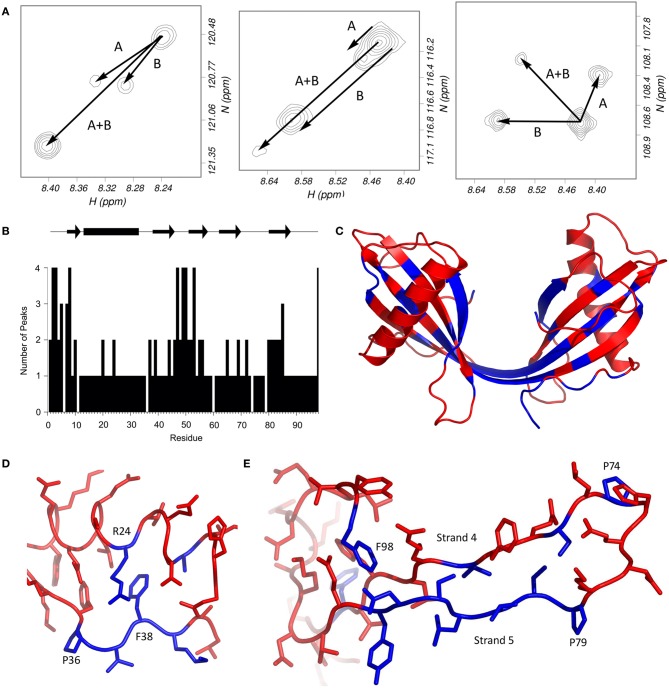
Figure 2.
Multiple HSQC peaks in the spectrum of the stefin B dimer. (A) Pattern of multiple peaks in the HSQC spectrum. To illustrate, the four peaks assigned to each of methionine 2 (left), serine 7 (centre) and glycine 50 (right) suggest that two conformational changes (labeled A and B) can account for the four states. (B) The number of peaks in the HSQC are plotted against residue number. (C) Residues with multiple peaks are colored blue in the structure of the dimer (bottom). (D) Multiple HSQC peaks around proline 36. In this view of the stefin B monomer (PDB code 1STF), residues with multiple peaks (and proline 36) are blue; other peaks are red. Proline 36 is in the trans conformation, and isomerization could affect the conformation of residues 37–39, as well as arginine 24, which packs against phenylalanine 38. The resulting two structures could give rise to the two peaks seen for each blue residue in the HSQC spectrum of the dimer. (E) Multiple HSQC peaks around strands 4 and 5. The loop between the two C-terminal β-strands of stefin B is part of the protease binding site, and is flanked by two proline residues at positions 74 and 79. Cis-trans isomerism in proline 79 could be the cause of the multiple peaks observed on strand 5, which could in turn affect phenylalanine 98. The two prolines, and residues which have multiple peaks in the HSQC spectrum of the dimer are blue; other residues are red.