Abstract
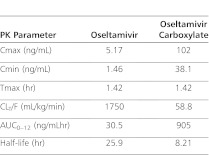
This report details the pharmacokinetics of oseltamivir and oseltamivir carboxylate following administration of high-dose oseltamivir in a critically ill child receiving extracorporeal membrane oxygenation (ECMO) and continuous venovenous hemodialysis (CVVHD). A 6-year-old critically ill male patient suffering from a presumed viral illness was transferred to our institution's pediatric intensive care unit from an outside hospital after developing respiratory failure and cardiomegaly. ECMO and oseltamivir therapy were initiated upon admission, and CVVHD was started on hospital day 3. Pharmacokinetic sampling occurred at an oseltamivir dose of approximately 4 mg/kg on hospital day 6. The patient's oseltamivir and oseltamivir carboxylate area under the plasma concentration time curves for the 12-hour dosing interval (AUC0–12) were 30.5 and 905 ng/mLhr, respectively. Drug clearance by CVVHD was 31.6 mL/min for oseltamivir and 26.9 mL/min for oseltamivir carboxylate. Pre- and postoxygenator oseltamivir and oseltamivir carboxylate plasma concentrations did not differ substantially. The patient's oseltamivir carboxylate plasma concentrations remained well above the reported mean 50% inhibitory concentration for 2009 pandemic H1N1 virus. However, despite receiving twice the standard dose of oseltamivir, the oseltamivir carboxylate AUC0–12 in our patient was less than that reported in noncritically ill pediatric subjects. The reduced oseltamivir carboxylate AUC0–12 found in our patient was most likely due to decreased drug absorption.
INDEX TERMS: continuous renal replacement therapy, dialysis, extracorporeal membrane oxygenation, influenza, oseltamivir
INTRODUCTION
Oseltamivir, an orally administered neuraminidase inhibitor, is indicated for the prophylaxis and treatment of illnesses related to influenza A and B infection.1 The pharmacokinetics of oseltamivir and its active metabolite, oseltamivir carboxylate, in critically ill children receiving extracorporeal therapies is largely unknown. Extracorporeal membrane oxygenation (ECMO) can alter the pharmacokinetics of some medications through increased total blood volume and drug adsorption to the tubing or oxygenator.2,3 Many children receiving ECMO also receive continuous renal replacement therapies (CRRTs), adding an additional source of drug clearance. One published case series detailed the pharmacokinetics of a 4 mg/kg dose of oseltamivir in three children who received ECMO and continuous venovenous hemofiltration (CVVH); however, oseltamivir binding to the ECMO oxygenator and the contribution of CVVH to oseltamivir clearance were not assessed.4 The present report is the first to detail the effects of ECMO and CVVHD on oseltamivir and oseltamivir carboxylate disposition in a critically ill child and to illustrate an instance of decreased oseltamivir exposure in this patient.
CASE REPORT
This was an open label pharmacokinetic investigation of oseltamivir pharmacokinetics in a critically ill 6-year-old male patient suffering from acute respiratory distress syndrome presumed to be secondary to a viral illness and receiving ECMO and CVVHD. The evaluation was institutional review board-approved and was part of a larger study that included adults.5 Informed consent was obtained from the patient's legally authorized guardian prior to enrollment. Oseltamivir therapy was started upon presentation to our institution at a dose of 45 mg (2 mg/kg) oral oseltamivir suspension every 12 hours. CVVHD was initiated on day 3. Therapeutic plasma exchange was also initiated on day 3 and was performed for two more sessions on days 4 and 5. On day 4 (after six oseltamivir doses of 45 mg), the oseltamivir dosage was increased to 90 mg (4 mg/kg) every 12 hours in order to extend the Centers for Disease Control and Prevention recommendation that a double dose of oseltamivir be considered in critically ill adult patients to the pediatric population in our institution.6
Pharmacokinetic sampling occurred on day 6, after the fourth dose of 90 mg (tenth overall dose at our institution). The 15-mg/mL oral oseltamivir suspension was compounded from oral capsules in accordance with published instructions.7 On the day of sampling, the patient did not require vasopressor support. He was receiving total parenteral nutrition due to lack of bowel sounds and was approximately 30% fluid overloaded, with a recent weight of 28.2 kg (estimated dry weight, 21.5 kg). He was receiving ECMO therapy at blood flow rates of 1.1 to 1.5 L/min. In order to evaluate drug adsorption to the ECMO oxygenator, blood samples were collected from the ECMO circuit into evacuated ethylenediaminetetraacetic acid/sodium fluoride tubes before and after the oxygenator at 1, 2, 4, and 12 hours after oseltamivir administration.
CVVHD was discontinued right before the oseltamivir study dose was given due to perceived clinical improvement. Due to increasing fluid overload, CVVHD was reinitiated at the 7-hour time point with an M60 AN69 hollow fiber filter (Gambro, Lakewood, CO) at a dialysate flow rate of 1000 mL/hr and a mean ultrafiltration rate of 340 mL/hr. In order to evaluate oseltamivir clearance by CVVHD, blood samples were collected from the sampling port of the CVVHD circuit located just before the hemodialyzer at 0, 7, 8, 10, and 12 hours. Effluent samples (spent dialysate and ultrafiltrate) were collected at the same time points from the effluent port of the CVVHD circuit into polypropylene cryovials. The total volume of urine produced during the interval was recorded, and a 5-mL aliquot was taken for analysis. Blood samples were centrifuged, and plasma was separated, split into cryovials, and frozen at −80° C.
Samples were shipped on dry ice to PRA International Bioanalytical Laboratory (Assen, The Netherlands) for analysis. Samples were assayed for both oseltamivir and oseltamivir carboxylate, using high-performance liquid chromatography with tandem mass spectrometry.
Noncompartmental methods were used for pharmacokinetic analysis. Prefilter plasma concentrations were adjusted to account for dilution by a prefilter citrate infusion given for regional anticoagulation.8 Renal drug clearance was calculated as urine concentration multiplied by urine volume/AUC0–12. Saturation coefficient (SA) was calculated as effluent concentration divided by the corresponding prefilter plasma concentration. Oseltamivir clearance due to CVVHD (CLCVVHD) was calculated as effluent rate multiplied by SA.
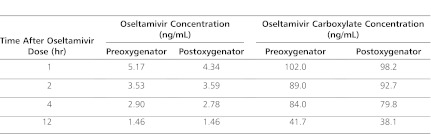
The oseltamivir and oseltamivir carboxylate concentrations drawn pre- and postoxygenator are presented in Table 1. For CVVHD therapy, the mean calculated SA ± SD was 0.99 ± 0.04 for oseltamivir and 0.85 ± 0.03 for oseltamivir carboxylate. Oseltamivir and oseltamivir carboxylate clearance values by CVVHD were 31.6 mL/min (1.1 mL/kg/min) and 26.9 mL/min (1 mL/kg/min), respectively. CVVHD removed a calculated 0.01 mg of oseltamivir and 0.55 mg of oseltamivir carboxylate during the 5 hours that CVVHD was running. Renal clearance values of oseltamivir and oseltamivir carboxylate were 155.5 mL/min (5.51 mL/kg/min) and 107.3 mL/min (3.80 mL/kg/min), respectively. A total of 0.28 mg of oseltamivir and 5.83 mg of oseltamivir carboxylate were calculated to have been removed by the kidney during the study interval. Results of the noncompartmental pharmacokinetic analysis are presented in Table 2. The patient survived his illness and was transferred from the intensive care unit (ICU) and eventually discharged home.
Table 1.
Oseltamivir and Oseltamivir Carboxylate Plasma Concentrations (ng/mL) Before and After the ECMO Oxygenator
Table 2.
Calculated Pharmacokinetic Parameters
DISCUSSION
This report is the first to examine oseltamivir concentrations before and after the ECMO oxygenator and the first to quantify oseltamivir clearance by CVVHD in a child. The patient was receiving several extracorporeal therapies that could impact drug disposition, such as ECMO and CVVHD. The high SAs found for oseltamivir and oseltamivir carboxylate in this patient indicated that both parent drug and active metabolite easily crossed the hemodialysis filter. The differences between pre- and postoxygenator concentrations for both oseltamivir and oseltamivir carboxylate were small, suggesting little drug removal by the oxygenator during ECMO therapy.
Because the half-life of oseltamivir carboxylate was prolonged in this patient and because he had undergone the starting and stopping of several therapies that might have affected drug clearance (a course of plasmapheresis was completed on the day prior to the study, and CVVHD was restarted during the study interval), oseltamivir pharmacokinetic parameters were not calculated under steady-state conditions. Regardless, the patient's oseltamivir carboxylate plasma concentrations remained well above the mean 50% inhibitory concentration (IC50) of 0.186 ± 0.107 ng/mL reported for 2009 pandemic H1N1 virus.9 It is difficult to compare our pharmacokinetic findings to those reported in noncritically ill pediatric volunteers because studies used different oseltamivir dosages and reported AUC0–24 instead of AUC0–12. However, it is evident that the oseltamivir carboxylate exposure in our patient who received a 4 mg/kg dose (AUC0–12, 905 ng/mLhr) was smaller than that observed in published reports of noncritically ill pediatric subjects who received the standard 2 mg/kg oseltamivir dose (mean AUC0–24 of 2969 ng/mLhr).10 The reduced oseltamivir carboxylate AUC found in our patient could have been due to 1) increased drug clearance, 2) increased volume of distribution, and/or 3) decreased drug absorption compared to noncritically ill children. Increased drug clearance is unlikely to be the reason for the reduced AUC, as the patient's calculated renal clearance of oseltamivir carboxylate (3.8 mL/kg/min) was somewhat lower than that predicted for a 6-year-old subject with normal renal function (6.79 mL/kg/min),10 whereas CVVHD clearance only contributed an additional 1.0 mL/kg/min. Although the volume of distribution could have been increased by as much as 30% due to expanded blood volume as part of ECMO therapy and fluid overload, that degree of increased volume is probably not sufficient to explain the large difference in AUC in our patient versus that predicted in normal children. Given the fact that oseltamivir carboxylate freely crossed the hemodialysis membrane and was >99% renally excreted,1 decreased drug absorption is the best explanation for why renal and CVVHD clearance combined accounted for only 8% of total clearance/F (CLT/F). If oseltamivir carboxylate had a low bioavailability (i.e., a low F value) in this patient, then the calculated CLT/F would be elevated because of low absorption rather than a truly high clearance.
One other case of suspected decreased oral oseltamivir absorption in a subject has been reported as part of a case series of three pediatric patients receiving ECMO and CRRT.4 Although CVVHD, ECMO, and renal clearances were not calculated in that series, it was observed that one of the three patients exhibited decreased oseltamivir carboxylate exposures. Although adsorption to the ECMO oxygenator itself does not appear to be a substantial source of oseltamivir or oseltamivir carboxylate clearance based on our findings, our very limited data suggest that critically ill children receiving ECMO and CRRT may exhibit decreased drug exposures compared to those of noncritically ill children.
CONCLUSIONS
While oseltamivir and oseltamivir carboxylate crossed the dialyzer during CVVHD, CVVHD was not an important clearance mechanism in this child, who had some residual renal function. The ECMO oxygenator did not substantially contribute to overall drug clearance. AUC0–12 oseltamivir carboxylate concentrations were lower than expected based upon the available literature, although plasma concentrations were well above the mean IC50 reported for H1N1 virus throughout the dosing interval. Decreased oral bioavailability of oseltamivir is a plausible explanation for these pharmacokinetic findings.
ACKNOWLEDGMENTS
Rachel Eyler was a research fellow at University of Michigan when this study was conducted. ClinicalTrials.gov identifier: NCT01048879.
ABBREVIATIONS
- AUC
Area Under the concentration time Curve
- CLT
total clearance
- Cmax
maximum concentration
- Cmin
minimum concentration achieved during the dosing interval
- CRRT
Continuous Renal Replacement Therapy
- CVVHD
Continuous Veno-venous Hemodialysis
- ECMO
Extracorporeal Membrane Oxygenation
- F
Bioavailability
- ICU
Intensive Care Unit
- SA
Saturation Coefficient
- Tmax
time that maximum concentration was achieved
- Vss
volume of distribution at steady state
- IC50
half maximal inhibitory concentration
Footnotes
DISCLOSURE Roche Pharmaceuticals provided funding for this study. The authors declare no other conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Genentech. Tamiflu prescribing information. Genentech, USA; South San Francisco, CA: 2011. http://www.gene.com/gene/products/information/tamiflu/pdf/pi.pdf. Accessed July 14, 2012. [Google Scholar]
- 2.Wildschut ED, Ahsman MJ, Allegaert K, et al. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36(12):2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulla H, McCormack P, Lawson G, et al. Pharmacokinetics of midazolam in neonates undergoing extracorporeal membrane oxygenation. Anesthesiology. 2003;99(2):275–282. doi: 10.1097/00000542-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Wildschut ED, de Hoog M, Ahsman MJ, et al. Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0010938. :e10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyler RF, Heung M, Pleva M, et al. Oseltamivir and Oseltamivir Carboxylate Pharmacokinetics in Critically Ill Patients Receiving Continuous Venovenous Hemodialysis [abstract A1–1999] Proceedings of the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy. Boston, MA. September 15, 2010. [Google Scholar]
- 6.World Health Organization. WHO guidelines for clinical management of human infection with pandemic (H1N1) 2009: revised guidance. November 2009. http://www.who.int/csr/resources/publications/swineflu/clinical_management/en/index.html. Accessed July 14, 2012. [Google Scholar]
- 7.Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook, 16th ed. Hudson, OH: Lexi-Comp; 2009. eds. [Google Scholar]
- 8.Vilay AM, Grio M, DePestel DD, et al. Daptomycin pharmacokinetics in critically ill patients receiving continuous venovenous hemodialysis. Crit Care Med. 2011;39(1):19–25. doi: 10.1097/CCM.0b013e3181fa36fb. [DOI] [PubMed] [Google Scholar]
- 9.Gubavera L, Okomo-Adhiambo M, Deyde V, et al. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:433–435. [PubMed] [Google Scholar]
- 10.Oo C, Barrett J, Hill G, et al. Pharmacokinetics and dosage recommendations for an oseltamivir oral suspension for the treatment of influenza in children. Paediatr Drugs. 2001;3(3):229–236. doi: 10.2165/00128072-200103030-00005. 7. [DOI] [PubMed] [Google Scholar]