Abstract
Objective
White blood cells are not traditionally considered to be normally present in amniotic fluid. This study was conducted after the observation that a patient with preterm labor and intact membranes had eosinophils as a predominant cell in the amniotic fluid, and had an episode of asthma during the index pregnancy. The goal of this study was to determine whether women presenting with preterm labor with eosinophils in the amniotic fluid had a different outcome than those without eosinophils as the predominant white blood cell in the amniotic cavity.
Methods
This retrospective case-control study included women who presented with preterm labor and intact membranes between 24 and 34 weeks of gestation. Patients underwent an amniocentesis shortly after admission for the assessment of the microbiologic status of the amniotic cavity and/or fetal lung maturity. Amniotic fluid was cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas. Cytologic studies included amniotic fluid white blood cell count and differential, which was performed on cytocentrifuged specimens. Patients with microbial invasion of the amniotic cavity and/or a white blood cell count >20 cells/mm3 were excluded from the study. Cases were defined as women in whom the differential contained >20% of eosinophils. Controls were selected among women with an amniotic fluid eosinophil count ≤20% and matched for gestational age at amniocentesis. The analysis was conducted with non-parametric statistics.
Results
The study population consisted of 10 cases and 50 controls. Gestational age and cervical dilatation at admission were similar in both groups. Cases had a lower gestational age at delivery than controls [34.6 weeks, inter-quartile range (IQR) 32–37.3 weeks vs. 38.0 weeks, IQR 35–40 weeks, respectively; p=0.018]. The prevalence of preterm delivery ≤35 weeks was higher among patients who had >20% eosinophils than in the control group [50% (5/10) vs. 18% (9/50), respectively; p=0.029]. Similar results were observed for delivery at <37 weeks [Cases: 70% (7/10) vs. Controls: 36% (18/50); p=0.046].
Conclusions
Women with preterm labor and intact membranes who have a large proportion of eosinophils in the amniotic fluid are at an increased risk for spontaneous preterm delivery. These patients may have had an episode of preterm labor related to a type I hypersensitivity reaction.
Keywords: Premature birth, preterm birth, prematurity, premature labor, mast cells, amniotic fluid cells, amniotic fluid white blood cells, allergy, allergy-induced preterm labor, atopy, pregnancy, type I hypersensitivity reaction, parturition, labor, eosinophil granule proteins
INTRODUCTION
Preterm parturition is a syndrome[1] caused by multiple etiologies, including intrauterine infection/inflammation,[2–24] uteroplacental ischemia,[25,26] cervical disorders,[27–29] uterine overdistension,[30] abnormal allograft reaction,[31] endocrine disorders,[32–35] and other causes. Some forms of preterm labor remain idiopathic in nature. In other words, no clear mechanism of disease can be implicated.
More than 20 years ago, one of the authors (RR) performed an amniocentesis in a patient presenting with preterm labor and intact membranes, and the laboratory reported that the predominant cell in the fluid were eosinophils. The laboratory staff believed that this was a mistake, and suspected that the fluid was erroneously labeled in the laboratory as amniotic fluid rather than tracheo-bronchial lavage, and requested that additional fluid be sent for reexamination. After explaining to the patient what had transpired, a second amniocentesis was performed and the laboratory confirmed that, in fact, eosinophils were the predominant cells in the amniotic fluid (Figure 1). Upon further conversation with the patient she expressed that developed asthma for the first time during this pregnancy. After this observation and consideration of the potential implications, a retrospective study was performed to determine the clinical significance of eosinophils in the amniotic cavity. This study was presented at the Society of Perinatal Obstetricians in 1991.[36] Since that time, the authors have received numerous calls from the United States and abroad, in which amniocenteses of patients in preterm labor have revealed eosinophils. We decided to share these results with the scientific community because there is no literature to provide guidance to physicians, and we have been asked to advice as to the clinical significance of this finding. This study was conducted to address this question.
Figure 1. Eosinophils in the amniotic fluid.
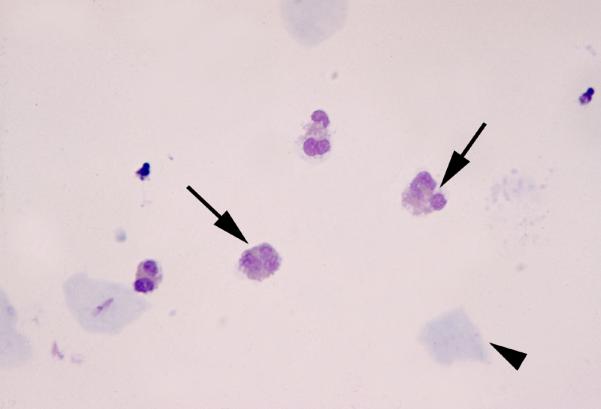
Amniotic fluid obtained from a patient with spontaneous preterm labor and intact membranes. The eosinophils (arrows) were the predominant cells in the amniotic fluid (arrowhead: epithelial cell).
MATERIALS AND METHODS
Study population
A retrospective case-control study was conducted by searching our perinatal database to identify women who were admitted with the diagnosis of preterm labor with intact membranes and met the following criteria: 1) singleton gestation; 2) gestational age between 24 and 34 completed weeks; and 3) amniocentesis with a negative culture, a white blood cell count of <20 cells/mm3 and a differential available. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical change before 37 completed weeks of gestation that required hospitalization. Women were cared for at Hutzel Hospital, Detroit, Michigan. Cases were women in which the differential showed that eosinophils represented >20% of the white blood cells in the amniotic fluid. Controls were patients in which the amniotic fluid differential contained ≤20% of eosinophils. Patients were matched for gestational age at the time of amniocentesis. All women provided written informed consent prior to the collection of amniotic fluid. The collection and utilization of amniotic fluid for research purposes was approved by the Institutional Review Boards of the participant institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Amniotic fluid studies
Amniotic fluid was obtained by transabdominal amniocentesis under ultrasonographic guidance. The fluid was transported to the laboratory in a capped plastic syringe and cultured for aerobic and anaerobic bacteria, as well as for mycoplasmas. White blood cell count, glucose concentration and Gram-stain were also performed shortly after collection. Cytologic studies included cell count and differential, which was performed by trained, technical personnel in the laboratory at the Detroit Medical Center. Cytocentrifuged smears of amniotic fluid stained with Wright-Giemsa were used for this purpose. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C and the supernatant was aliquoted and stored at - 70°C until analysis.
Statistical analysis
The normality of the data was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Comparisons between proportions were performed with the Chi-square test, and Mann-Whitney U tests were used for continuous variables. A Kaplan-Meier survival analysis was conducted to assess the amniocentesis-to-delivery interval. Spontaneous labor was entered in the analysis as the event of interest, and patients who delivered due to fetal or maternal indications were treated as censored observations with a censoring time equal to the amniocentesis-to-delivery interval.
RESULTS
The study population consisted of 10 cases and 50 controls. Table I displays the clinical characteristics of the study population. No differences were observed in the median gestational age at amniocentesis (30.4 weeks vs. 30 weeks, respectively; p=0.7) and median cervical dilatation at admission (1.5 cm vs. 1.0 cm, respectively; p=0.5) between cases and controls.
Table I.
Clinical characteristics of the study population
| Cases (n=10) | Controls (n=50) | p | |
|---|---|---|---|
| Nullipara | 20 (2/10) | 32 (16/50) | 0.5 |
| Previous preterm delivery | 10 (1/10) | 20 (10/50) | 0.5 |
| Gestational age at amniocentesis (wks) | 30.4 (27–34.1) | 30 (28.6–31.3) | 0.7 |
| Cervical dilatation at admission (cm) | 1.5 (1–3) | 1.0 (1–3) | 0.6 |
| AF WBC count (cells/mm3) | 6 (5–8) | 2 (0–9) | 0.019 |
| AF glucose (mg/dL) | 28 (25–43) | 36 (28–42) | 0.3 |
| Gestational age at delivery (wks) | 34.6 (32–37.3) | 38.0 (35–40) | 0.018 |
| Preterm delivery ≤ 35 weeks | 50 (5/10) | 18 (9/50) | 0.029 |
| Preterm delivery ≤ 37 weeks | 70 (7/10) | 36 (18/50) | 0.046 |
| Birthweight (grs) | 2,048 (1,860–3,033) | 2,693 (2,011–3,125) | 0.1 |
Results are expressed as median (inter-quartile range) and percentage (proportion) AF: amniotic fluid; WBC: white blood cells
Patients with more than 20% eosinophils in the amniotic fluid white blood cells differential count had a lower median gestational age at delivery than controls [Cases: 34.6 weeks, inter-quartile range (IQR) 32–37.3 weeks vs. Controls: 38.0 weeks, IQR 35–40 weeks; p0.018]. The frequency of preterm birth before 35 weeks of gestation was higher among cases than in controls [Cases: 50% (5/10) vs. Controls: 18% (9/50); p=0.029]. Similar results were observed for delivery at less than 37 weeks of gestation [Cases: 70% (7/10) vs. Controls: 36% (18/50); p=0.046].
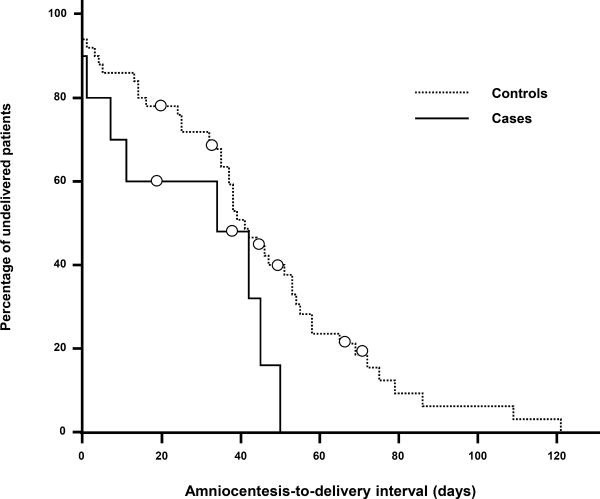
Although the amniocentesis-to-delivery interval was not significantly different, the median difference between cases and controls was 7 days [Cases: 34 days (95% CI 0–70 days) vs. Controls: 41 days (95% CI 34–48 days); p=0.084] (Figure 2).
Figure 2. Amniocentesis-to-delivery interval according to the presence or absence of amniotic fluid white blood cell count differential of more than 20% of eosinophils.
Although the difference was not significant, the median amniocentesis-to-delivery interval was seven days shorter in cases than controls [Cases: 34 days (95% CI 0–70 days) vs. Controls: for cases and 41 days (95% CI 34–48 days); p=0.084]. Solid line: cases; dashed line: controls; open circles: censored patients.
COMMENTS
Principal Findings of the Study
1) A subset of patients with preterm labor and intact membranes have eosinophils in the amniotic fluid; 2) patients with an amniotic fluid white blood cell count differential containing more than 20% of eosinophils are at an increased risk of preterm delivery; 3) some patients with eosinophilis in amniotic fluid delivered at term and had no evidence of complications.
White Blood Cells in the Amniotic Fluid
Under normal circumstances, clinical laboratories do not report white blood cells in the amniotic fluid obtained in the midtrimester or in patients undergoing amniocentesis at term for fetal lung maturity studies without contamination with maternal blood. The first study[37] in which we examined the clinical value of an amniotic white blood cell count and differential as a rapid method for the diagnosis of microbial invasion of the amniotic cavity, and also for the detection of intra-amniotic inflammation, indicated that the most frequent cell was granulocytes, and specifically, neutrophils. We observed that some patients had lymphocytes, and others had macrophages. Yet, eosinophils were not described in that report (195 patients), which was conducted over a 31-month period at Yale-New Haven Hospital/Yale University.[37]
The potential importance of eosinophils in the amniotic fluid came to our attention with the case described in the introduction of this manuscript, and which justified the current study. The patient admitted with preterm labor and intact membranes and had eosinophils in the amniotic fluid as a predominant cell, presented after that study[37] was completed. After that time, we conducted a study to systematically examine the clinical significance of eosinophils in the amniotic fluid of patients with preterm labor and intact membranes. This study was presented at the Society for Perinatal Obstetricians in 1991,[36] and we observed that women with eosinophils were more likely to have a history of atopy (e.g. allergic rhinitis, atopic dermatitis, and 2 with asthma for the first time in the index pregnancy). The data from that study was misplaced by staff working at another institution located at Washington, D.C. Since eosinophils are elevated in peripheral blood and other biological fluids in the context of allergic reactions, it was proposed that eosinophils in the amniotic fluid may represent evidence of an “allergic-like” response in the uterus.[36] A few patients that we studied at Yale-New Haven Hospital and who were found to have eosinophils in the amniotic fluid had their stool examined for ova and parasites, and the results were negative. Therefore, we have no evidence of helminthic infections in these patients.
Eosinophils
These cells are bone-marrow derived, multi-functional leukocytes, actively motile, and terminally differentiated (e.g. non-dividing cells).[38] The nucleus of the cell is bi-lobed, and the cytoplasm contains specific granules which have arginine-rich basic proteins, which are colored bright orange by acidic dyes such as eosin.[39] This is what gives eosinophils their name and typical appearance under the microscope. The staining properties of eosinophils were discovered by Paul Ehrlich and presented to the Berlin Physiologic Society in 1878.[40] The cytoplasm of eosinophils contains primary granules, secondary granules, lipid bodies, and Charcot-Leyden crystals, which are only seen during activation.[41] The primary granules are the main site of Charcot-Leyden crystal protein, which is also called galectin-10. This glycoprotein accounts for 10% of the total cellular proteins in the mature blood eosinophil. The secondary granule contains major basic protein (MBP), eosinophilic cationic protein (ECP), eosinophil peroxidase (EPO), eosinophil-derived neurotoxin (EDN), as well as several cytokines.[42]
Eosinopoiesis occurs mainly in the bone marrow, and other sites of production include the spleen, the thymus, and the lymph nodes.[43] The post-mitotic eosinophil reservoir capacity is approximately 9–14 × 108 cells per kilogram.[44] The development of multipotential cells into eosinophil progenitors is thought to result from a stochastic process,[43] followed by survival which is supported by several cytokines including interleukin (IL)-3, IL-4, IL-5, stem cell factor, granulocyte-macrophage colony stimulating factor (GM-CSF), and eotaxin. These cytokines sustain the terminal stages of eosinophil maturation and release into the bloodstream.[45,46] The bone marrow of healthy individuals contains about 3% of eosinophils, of which 37% are mature, up to 16% of eosinophilic myelocytes are in the S-phase of the cell cycle (which lasts approximately 13 hours). The time elapsed between the last mitosis and the appearance of eosinophils in blood as mature cells is approximately 2.5 to 3.5 days.[42,44,47] The average residence of an eosinophil in the bone marrow is 3–5 days. The half-life of eosinophils in the circulation is about 18 hours, and the mean blood transit time is approximately 26 hours.[48]
Most of the total eosinophil pool (99%) is contained in connective tissues, and eosinophils represent less than 4% of circulating white blood cells.[41] Circulating eosinophils migrate into tissues to exert their biological properties and participate in inflammatory processes elicited by parasitic helminth infections, and allergic diseases.[49,50] Eosinophils are normal constituents of the mucosal immune system of the gastrointestinal, respiratory, and urogenital tracts.[49]
Eosinophils are derived from a pluripotent stem cell, which differentiates into a common precursor of basophils and eosinophils. Then, there is further differentiation into a separate eosinophil lineage.[51] Three cytokines play a role in regulating eosinophilic development (sometimes, they are collectively referred to as “eosinopoietins”): IL-3, IL-5, and GM-CSF.[52–55] IL-5 is the most specific to the eosinophil lineage, can stimulate the release of eosinophils from the bone marrow into the peripheral circulation,[56]and transgenic mice for IL-5 results in substantial eosinophilia.[57–60] In addition to the role of IL-5, the most important chemokine that regulates the traffic of eosinophils into tissues under physiologic conditions is eotaxin-1.[49] This chemokine and its receptor are important under physiologic conditions for the delivery of eosinophils to the mucosa of the gastrointestinal tract, mammary gland, thymus, and uterus (endometrium).[50,61–67]
The role of eosinophils in the endometrium is believed to be related to estrus cycling.[49,68] Indeed, eotaxin-1 peripheral concentrations change in response to estrogens, and the number of eosinophils in the endometrium changes during the menstrual cycle.[38,50] Moreover, gene deletion of IL-5 decreases the number of eosinophils in the blood,[69,70] as well as in the endometrium (4 to 7-fold) in the estrus cycle and in early pregnancy.[66] Similarly, eosinophils in the cervix and decidua are decreased in IL-5 knockout mice. Yet, eosinophils have been found in the endometrium of these animals, suggesting that a subpopulation of eosinophils is independent of IL-5. Interestingly, the onset of parturition is not affected in IL-5 knockout mice.[66]
Under pathologic conditions, several chemokines and cytokines are involved in the migration of eosinophils to inflammatory sites.[38,49,50,71] Eosinophils exert part of their biological properties through the release of proteins from intracellular granules. These proteins, called eosinophil-granule proteins, include: 1) major basic protein; 2) eosinophilic cationic protein; 3) eosinophilic peroxidase; and 4) eosinophilic-derived neurotoxin. These proteins are stored in the specific granules, and they are toxic to parasite larvae and to some mammalian cells. ECP and EDN are ribonucleases, and have anti-viral activity. ECP can induce mast cell degranulation, and suppress T-cell proliferative responses. MBP also induces mast cell and basophil degranulation. EPO constitutes 25% of the total protein mass of specific granules, and is involved in the formation of reactive oxygen species and reactive nitrogen metabolites. Consequently, they can be involved in promoting oxidative stress and cell death.[49] Eosinophilic proteins are considered to be almost exclusive to these cells. Plasma concentrations of MBP are 10 to 20 times higher in pregnant than in non-pregnant women.[72] Moreover, a relationship was reported between MBP concentrations and the impending onset of labor at term (in one case in preterm labor).[72] Since eosinophil numbers do not change during pregnancy, an alternative source of these proteins was searched and found in the placenta.[73,74] A role for MBP in labor was postulated because this protein has been localized to interstitial trophoblasts, which are in close proximity to the myometrium. It has been postulated that these cells may play a role in the onset of labor because of their proximity to the myometrium.[75]
Recently, a condition known as “eosinophilic/T-cell chorionic vasculitis” was described.[76] This condition is characterized by focal infiltration of chorionic vessels (artery or vein) by eosinophils, but its clinical significance remains to be determined. Redline reported that recurrent villitis associated with bacterial bacilli (demonstrated by Steiner stain) included macrophages, lymphocytes, plasma cells and neutrophils, as well as eosinophils.[77] Indeed, eosinophils can be observed in cases of acute chorioamnionitis. Eosinophils have been demonstrated in the placenta of animals having adverse pregnancy outcome. One case included preterm birth in an alpaca (Lama pacos) which delivered at 290 days of gestation (normal gestation 335–360 days) and had placentitis with a cellular infiltration by lymphocytes, eosinophils and neutrophils in the chorionic membranes.[78] Gram negative, period acid-Schiff positive, variably acid-fast spores were observed. Using molecular microbiologic techniques, encephalitozoon cuniculi-associated placentitis was identified using PCR and sequencing of the PCR products.[78] A second case of an infection in an alpaca was reported shortly after the animal had a spontaneous abortion.[79] In this case, the mother died after the abortion, and the autopsy showed disseminated eosinophilic myositis. Large cysts were observed and attributed to an infection with Sarcocystis sp. (probably Sarcocystis aucheniae); however, this organism was not cultured. There was no evidence of infection in the placenta or uterus.[79]
Eosinophils in the Amniotic Fluid
It is not known whether eosinophils in the amniotic fluid are of fetal or maternal origin. Similarly, the chemotactic stimulus that brings eosinophils into the amniotic fluid has not been identified and their role in the amniotic cavity is not known. Eosinophils have an important role against viruses[80–84] and it is possible that these cells participate in host-defense against viral infections. However, the study of the viral burden and diversity in amniotic fluid is in its infancy. We would like to emphasize that the purpose of this report is simply to call attention to the unexpected observation of finding eosinophils in the amniotic fluid. We have performed this study using the results reported by the clinical laboratory of the Detroit Medical Center during the course of clinical care. Therefore, eosinophils were identified in a routine cytocentrifuged smear. We have not conducted an eosinophil count, which has been used in the study of eosinophil biology and the diagnosis of hypereosinophilic syndromes.[85] Similarly, we are cautious in interpreting the biological meaning of our results.
Could Amniotic Fluid Eosinophils be a Marker for an Inflammatory Reaction and if So, What Type of Inflammatory Reaction?
Eosinophils are involved in the late phase of a Type I Hypersensitivity reaction (or allergic reaction).[86] Eosinophils are capable of creating an environment that stimulates mast cell degranulation (eosinophils incubated with the mast cell protease “chymase” can induce the production of “eosinophil-derived stem cell factor”, which is capable of promoting the growth of mast cells). Moreover, they are considered to be responsible for tissue damage that occurs during Type I Hypersensitivity reaction. Eosinophils can secrete collagenases, proteases, and peroxidases, and they may participate in tissue damage within the amniotic cavity. The arguments and evidence that a Type I Hypersensitivity reaction may participate as a cause of preterm labor have been described elsewhere (Romero R. et al., submitted). Whether or not patients with eosinophils in amniotic fluid are the same as those having an episode of preterm labor induced by an allergen remain to be determined.
Some Mysteries About Eosinophils
The traditional view is that the toxic products of eosinophils contribute to the killing of helminths, a view that can be traced back to the observation that the schistosomula of Schistoma mansoni can undergo eosinophilic–dependant killing.[87] Although eosinophils have been traditionally considered to provide protection to the host during parasitic infections, a recent study has called for a reexamination of this idea. This year, Fabre et al.[88] published a study in which eosinophils were required for the survival of the parasite. The experiments were conducted with Trichinella spiralis, a worm that is naturally found in mice, and produces a chronic infection in skeletal muscle. The parasite was introduced into wild-type mice as well as two strains lacking eosinophils. These cells were found to infiltrate infected muscles in wild-type mice. In contrast, Trichinella spiralis larvae die in large numbers in mice without eosinophils. The authors suggest that eosinophils may be important in establishing a chronic infection that would allow survival of the worm in the host.[88] Whether this is the case in humans as well as for other helminths remains to be determined.
The other major role assigned to eosinophils is to participate in allergic reactions.[38] In this inflammatory process, eosinophils have been considered to play a secondary role to helper type 2 (Th2) immune responses.[89] Yet, recent studies conducted with mice devoid of eosinophils indicate that Th2 cytokines are lower in these animals than in controls when challenged with an allergen.[90] These observations suggest that in the lung, eosinophils may be required for localized recruitment of effector T cells. If this is correct, then the role of eosinophils in the initiation of a Type I Hypersensitivity reaction would also need to be re-examined.
The phylogeny of eosinophils is also interesting. All vertebrates appear to have them. Eosinophils have been found in fish, amphibians, birds, and reptiles. In many of these classes, the eosinophil granules appear somewhat different in that they do not contain a crystalloid internum.[91] The precise role of this cell in these species remains to be determined.
Several investigators have reported the presence of eosinophilic proteins, such as ECP, in human amniotic fluid.[92–94] The sources and physiologic role of these proteins remains to be determined. Recently, the proform of eosinophil major basic protein (ProMBP) has been demonstrated to exist in the serum from pregnant women (and also in non-pregnant women) complexed with a variable fraction of angiotensinogen. Moreover, a subfraction binds to complement C3dg.[95] Yet, the clinical significance of ProMBP is unknown.
Recently, eosinophils have been proposed to play a role in innate immunity by recognizing the presence of “danger signals”.[96] The danger theory of immunity was proposed by Matzinger,[97] and postulates that the immune system recognizes molecules and cells capable of causing danger to the host rather than to discriminate between self and non-self. Therefore, these molecules can arise not only from microorganisms (non-self), but also from the host owned cells, which are damaged or stressed. The typical case would be a cell in the process of necrosis. Recent observations suggest that eosinophils from healthy individuals can recognize danger signals (necrotic intestinal cells) because such material was able to induce chemotaxis of eosinophils and the release of eosinophil peroxidase.[96] This proposal is interesting because it assigns to the eosinophil a constructive role in immune defense, rather than a destructive role, which is the reputation that eosinophils have enjoyed for several decades. The precise role of these cells may be tissue repair, given that they can produce growth factors such as fibroblast growth factor, nerve growth factor, vascular endothelial growth factor, and TGF-β1.[98–101] Since eosinophils express Toll-like receptor 4 as well as CD14, they can respond to bacterial endotoxin.[102,103] It is noteworthy that in the late 1800s, Paul Ehrlich had suggested that eosinophils could be activated by toxic products of foreign organisms or products of tissue breakdown.[104]
Eosinophilic Granulocytes and Damage-Associated Molecular Associated Patent Proteins (DAMPs)
Recent evidence suggests that eosinophils can act as antigen-presenting cells. Lucey et al.[105] demonstrated that eosinophils can express HLA-DR, and that this subset of cells can induce lymphocyte proliferation.[106] Moreover, adoptive transfer of antigen-pulsed eosinophils can induce antigen-specific T-cell responses in vivo.[106,107] An important discovery is that incubation of eosinophils with the high mobility group box 1 protein (HMGB1) induces up-regulation of HLA-DR and CD86. HMGB1 is a highly conserved protein present in the nuclei and cytoplasm of nearly all cell types, which is considered upon release of necrotic, but not apoptotic death of normal cells. HMGB1 is released by several immune and non-immune cells. HMGB1 is considered a “danger signal” for eosinophils. The administration of this protein to normal animals causes a systemic inflammatory response, including fever, acute lung injury, epithelial barrier dysfunction and death. Anti-HMGB1 treatment can rescue mice from lethal endotoxemia, or sepsis.[108] HMGB1 is a constituent of chromatin proteins and has chemotactic properties. Thus, Lotfi et al.[42] have argued that HMGB1 is ideally positioned to be a unique “danger signal” which identifies cells of regions of cellular stress/necrosis and attract granulocytes, including neutrophils. Indeed, HMGB1 can attract eosinophils, serve as a survival factor by itself, or in combination with eotaxin.[42] We believe that the expression of DAMPs within the amniotic cavity or in the uterine cavity may explain the influx of eosinophils in patients without infection. DAMPs may be induced by non-microbial related agents, under conditions of hypoxia or oxidative stress. This proposal provides a link between the presence of eosinophils in the amniotic fluid and a wide range of insults within the amniotic cavity.
Finally, eosinophils can also serve an immunoregulatory role. These cells can produce a number of cytokines, such as interferon gamma (Th-1), or members of the Th-2 family, such as IL-4, IL-5, IL-13. In addition, it can produce members of the Th-3 family, such as TGF-β and IL-10, and possibly, members of the Th-4 family of cytokines which includes IL-17, also known as Th-17.[109–111] Another role for eosinophils is to modulate local T-cell activity. This can be accomplished by the production of eosinophil-derived indoleamiane 2,3-dioxygenase (IDO) production of kynurenine.[112]
The Biological Complexity of Eosinophils
The traditional view that eosinophils were predominantly involved in helminth and parasite diseases has given way to a different view in which these cells play a more complex role in tissue hemostasis. Eosinophils have been implicated in the pathogenesis of a very long list of disease states, including autoimmune diseases, and even cancer. Lotfi et al.[42] has proposed that the current confusion about the role of eosinophils derived from the fact that these cells have evolved as part of host inflammatory/remodeling mechanism, and therefore, the specific role of these cells depends upon circumstances and that their primary function is to maintain tissue hemostasis.
Conclusion
Eosinophils can be found as the predominant cell in the amniotic fluid of a small subset of women with preterm labor and intact membranes. These patients are at increased risk for preterm delivery. Yet, many experience an episode of preterm labor and are discharged and deliver at term. Further studies are required to determine the role of eosinophils in the amniotic fluid, as well as the physiology of proteins which have been traditionally considered as eosinophilic-specific.
Acknowledgment
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R. Prenatal medicine: The child is the father of the man. J.Mat.Fet.Neonat.Med. 2009 doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 2.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin.Obstet.Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 3.Minkoff H. Prematurity: infection as an etiologic factor. Obstet.Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 4.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin.Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 6.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am.J.Obstet.Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 8.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment.Retard.Dev.Disabil.Res.Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern.Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 11.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet.Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 12.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, Khalek N, Camacho N, Hendler I, Mittal P, et al. Clinical significance of the presence of amniotic fluid 'sludge' in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet.Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, Kim CJ, Khalek N, Mittal P, Goncalves LF, et al. What is amniotic fluid 'sludge'? Ultrasound Obstet.Gynecol. 2007;30:793–798. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Espinoza J, Chaiworapongsa T, Gonzalez R, Iams JD, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern.Fetal Neonatal Med. 2007;20:167–173. doi: 10.1080/14767050601135485. [DOI] [PubMed] [Google Scholar]
- 15.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern.Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, Nhan-Chang CL, Erez O, Kim CJ, Espinoza J, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet.Gynecol. 2008;198:135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bujold E, Romero R, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Gomez R, Espinoza J, Vaisbuch E, Mee KY, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern.Fetal Neonatal Med. 2008;21:697–713. doi: 10.1080/14767050802053289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiworapongsa T, Hong JS, Hull WM, Romero R, Whitsett JA. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern.Fetal Neonatal Med. 2008;21:663–670. doi: 10.1080/14767050802215664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern.Fetal Neonatal Med. 2008;21:449–461. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, Vaisbuch E, Than NG, Mazaki-Tovi S, Chaiworapongsa T, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern.Fetal Neonatal Med. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern.Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern.Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, Mittal P, Kim CJ, Kim MJ, Espinoza J, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern.Fetal Neonatal Med. 2008;21:763–775. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Espinoza J, Rogers WT, Moser A, Nien JK, Kusanovic JP, Gotsch F, Erez O, Gomez R, Edwin S, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern.Fetal Neonatal Med. 2008;21:367–388. doi: 10.1080/14767050802045848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am.J.Obstet.Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 26.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet.Gynecol. 1997;89:265–271. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 27.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–1103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 28.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet.Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet.Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol. 1990;10:347–350. [PubMed] [Google Scholar]
- 31.Romero R, Sepulveda W, Baumann P, Yoon BH, Brandt F, Gomez R, Mazor M, Sorokin Y, Cotton D. The preterm labor syndrome: Biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease. Am J.Obstet.Gynecol. 1993;168:288. [Google Scholar]
- 32.Csapo AI, Pohanka O, Kaihola HL. Progesterone deficiency and premature labour. Br.Med.J. 1974;1:137–140. doi: 10.1136/bmj.1.5899.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Check JH, Lee G, Epstein R, Vetter B. Increased rate of preterm deliveries in untreated women with luteal phase deficiencies. Preliminary report. Gynecol.Obstet.Invest. 1992;33:183–184. doi: 10.1159/000294877. [DOI] [PubMed] [Google Scholar]
- 34.Mazor M, Hershkovitz R, Chaim W, Levy J, Sharony Y, Leiberman JR, Glezerman M. Human preterm birth is associated with systemic and local changes in progesterone/17 beta-estradiol ratios. Am.J.Obstet.Gynecol. 1994;171:231–236. doi: 10.1016/0002-9378(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 35.Fidel PI, Jr., Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J.Matern.Fetal Med. 1998;7:222–226. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Mazor M, Avila C, Quintero R, Munoz H. Uterine “allergy”: A novel mechanism for preterm labor. Am.J.Obstet.Gynecol. 1991;164:375. [Google Scholar]
- 37.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet.Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 38.Church MK, Lichtenstein LM, Simon HU, Wardlaw AJ, Haslett C, Lee TH, Hawrylowicz CM. Effector cells of allergy. Second Edition 2001. pp. 303–324. [Google Scholar]
- 39.Murphy K, Travers P, Walport M. Allergy and Hypersensitivity. Seventh Edition 2008. pp. 555–598. [Google Scholar]
- 40.Ehrlich P. Ueber die specifischen granulationen des blutes. Arch.Anat.Physiol. 1879:571–579. [Google Scholar]
- 41.Cells and tissues of the immune response. First Edition 2006. pp. 35–67. [Google Scholar]
- 42.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 43.TILL JE, MCCULLOCH EA, SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc.Natl.Acad.Sci.U.S.A. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parwaresch MR, Walle AJ, Arndt D. The peripheral kinetics of human radiolabelled eosinophils. Virchows Arch.B Cell Pathol. 1976:57–66. doi: 10.1007/BF02899144. [DOI] [PubMed] [Google Scholar]
- 45.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 46.Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5: the role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp.Med. 1998;188:1621–1632. doi: 10.1084/jem.188.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spry CJ, Kay AB, Gleich GJ. Eosinophils 1992. Immunol.Today. 1992;13:384–387. doi: 10.1016/0167-5699(92)90085-L. [DOI] [PubMed] [Google Scholar]
- 48.Steinbach KH, Schick P, Trepel F, Raffler H, Dohrmann J, Heilgeist G, Heltzel W, Li K, Past W, van der Woerd-de Lange JA, et al. Estimation of kinetic parameters of neutrophilic, eosinophilic, and basophilic granulocytes in human blood. Blut. 1979;39:27–38. doi: 10.1007/BF01008072. [DOI] [PubMed] [Google Scholar]
- 49.Rothenberg ME, Hogan SP. The eosinophil. Annu.Rev.Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 50.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin.Exp.Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 51.Boyce JA, Friend D, Matsumoto R, Austen KF, Owen WF. Differentiation in vitro of hybrid eosinophil/basophil granulocytes: autocrine function of an eosinophil developmental intermediate. J Exp.Med. 1995;182:49–57. doi: 10.1084/jem.182.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez AF, Begley CG, Williamson DJ, Warren DJ, Vadas MA, Sanderson CJ. Murine eosinophil differentiation factor. An eosinophil-specific colony-stimulating factor with activity for human cells. J Exp.Med. 1986;163:1085–1099. doi: 10.1084/jem.163.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp.Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothenberg ME, Pomerantz JL, Owen WF, Jr., Avraham S, Soberman RJ, Austen KF, Stevens RL. Characterization of a human eosinophil proteoglycan, and augmentation of its biosynthesis and size by interleukin 3, interleukin 5, and granulocyte/macrophage colony stimulating factor. J Biol.Chem. 1988;263:13901–13908. [PubMed] [Google Scholar]
- 55.Takatsu K, Takaki S, Hitoshi Y. Interleukin-5 and its receptor system: implications in the immune system and inflammation. Adv.Immunol. 1994;57:145–190. doi: 10.1016/s0065-2776(08)60673-2. [DOI] [PubMed] [Google Scholar]
- 56.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp.Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp.Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tominaga A, Takaki S, Koyama N, Katoh S, Matsumoto R, Migita M, Hitoshi Y, Hosoya Y, Yamauchi S, Kanai Y, et al. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp.Med. 1991;173:429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp.Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mishra A, Hogan SP, Brandt EB, Wagner N, Crossman MW, Foster PS, Rothenberg ME. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol.Chem. 2002;277:4406–4412. doi: 10.1074/jbc.M110424200. [DOI] [PubMed] [Google Scholar]
- 61.Casslen B, Kobayashi TK, Stormby N. Cyclic variation of the cellular components in human uterine fluid. J Reprod.Fertil. 1982;66:213–218. doi: 10.1530/jrf.0.0660213. [DOI] [PubMed] [Google Scholar]
- 62.Honda K, Chihara J. Eosinophil activation by eotaxin--eotaxin primes the production of reactive oxygen species from eosinophils. Allergy. 1999;54:1262–1269. doi: 10.1034/j.1398-9995.1999.00170.x. [DOI] [PubMed] [Google Scholar]
- 63.Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. Mol.Med.Today. 2000;6:20–27. doi: 10.1016/s1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- 64.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Lathbury LJ, Salamonsen LA. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol.Reprod. 2000;62:404–411. doi: 10.1095/biolreprod62.2.404. [DOI] [PubMed] [Google Scholar]
- 66.Robertson SA, Mau VJ, Young IG, Matthaei KI. Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. J Reprod.Fertil. 2000;120:423–432. doi: 10.1530/jrf.0.1200423. [DOI] [PubMed] [Google Scholar]
- 67.Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142:4515–4521. doi: 10.1210/endo.142.10.8459. [DOI] [PubMed] [Google Scholar]
- 68.Spry CJF. Female genital tract diseases and pregnancy. First Edition 1988. pp. 226–227. [Google Scholar]
- 69.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 70.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp.Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spry CJF. Methods: eosinophil structure, constituents, and metabolism. First Edition 1988. pp. 29–73. [Google Scholar]
- 72.Wasmoen TL, Coulam CB, Leiferman KM, Gleich GJ. Increases of plasma eosinophil major basic protein levels late in pregnancy predict onset of labor. Proc.Natl.Acad.Sci.U.S.A. 1987;84:3029–3032. doi: 10.1073/pnas.84.9.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maddox DE, Kephart GM, Coulam CB, Butterfield JH, Benirschke K, Gleich GJ. Localization of a molecule immunochemically similar to eosinophil major basic protein in human placenta. J Exp.Med. 1984;160:29–41. doi: 10.1084/jem.160.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wasmoen TL, McKean DJ, Benirschke K, Coulam CB, Gleich GJ. Evidence of eosinophil granule major basic protein in human placenta. J Exp.Med. 1989;170:2051–2063. doi: 10.1084/jem.170.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagner JM, Hustin J, Bonno M, Kephart GM, Gurian KV, Gleich GJ. Pregnancy-associated major basic protein: deposition of protein and expression of mRNA at the maternal-fetal junction in early and late gestation. Placenta. 1994;15:625–640. doi: 10.1016/s0143-4004(05)80409-6. [DOI] [PubMed] [Google Scholar]
- 76.Fraser RB, Wright JR., Jr. Eosinophilic/T-cell chorionic vasculitis. Pediatr.Dev.Pathol. 2002;5:350–355. doi: 10.1007/s10024-001-0128-9. [DOI] [PubMed] [Google Scholar]
- 77.Redline RW. Recurrent villitis of bacterial etiology. Pediatr.Pathol.Lab Med. 1996;16:995–1001. doi: 10.1080/15513819609168723. [DOI] [PubMed] [Google Scholar]
- 78.Webster JD, Miller MA, Vemulapalli R. Encephalitozoon cuniculi-associated placentitis and perinatal death in an alpaca (Lama pacos) Vet.Pathol. 2008;45:255–258. doi: 10.1354/vp.45-2-255. [DOI] [PubMed] [Google Scholar]
- 79.La Perle KM, Silveria F, Anderson DE, Blomme EA. Dalmeny disease in an alpaca (Lama pacos): sarcocystosis, eosinophilic myositis and abortion. J Comp Pathol. 1999;121:287–293. doi: 10.1053/jcpa.1999.0321. [DOI] [PubMed] [Google Scholar]
- 80.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect.Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 81.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp.Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc.Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 83.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 84.Rosenberg HF, Dyer KD, Domachowske JB. Eosinophils and their interactions with respiratory virus pathogens. Immunol.Res. 2009;43:128–137. doi: 10.1007/s12026-008-8058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gleich GJ, Leiferman KM. The hypereosinophilic syndromes: current concepts and treatments. Br.J Haematol. 2009;145:271–285. doi: 10.1111/j.1365-2141.2009.07599.x. [DOI] [PubMed] [Google Scholar]
- 86.Allergy and Hypersensitivity. First Edition 2006. pp. 923–962. [Google Scholar]
- 87.Butterworth AE, Sturrock RF, Houba V, Mahmoud AA, Sher A, Rees PH. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- 88.Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin.Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 90.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp.Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spry CJF. Eosinophils in animals other than man. First Edition 1988. pp. 123–127. [Google Scholar]
- 92.Vernof KK, Ory SJ, Gleich GJ. Pregnancy-associated major basic protein in amniotic fluid. J Reprod.Immunol. 1992;21:47–56. doi: 10.1016/0165-0378(92)90039-7. [DOI] [PubMed] [Google Scholar]
- 93.Rice GE, Reimert CM, Bendtzen K. Eosinophil cationic protein and eosinophil protein X: human amniotic fluid concentrations and gestational tissue content at term. Placenta. 1998;19:181–185. doi: 10.1016/s0143-4004(98)90007-8. [DOI] [PubMed] [Google Scholar]
- 94.Christiansen M, Jaliashvili I, Overgaard MT, Ensinger C, Obrist P, Oxvig C. Quantification and characterization of pregnancy-associated complexes of angiotensinogen and the proform of eosinophil major basic protein in serum and amniotic fluid. Clin.Chem. 2000;46:1099–1105. [PubMed] [Google Scholar]
- 95.Oxvig C, Haaning J, Kristensen L, Wagner JM, Rubin I, Stigbrand T, Gleich GJ, Sottrup-Jensen L. Identification of angiotensinogen and complement C3dg as novel proteins binding the proform of eosinophil major basic protein in human pregnancy serum and plasma. J Biol.Chem. 1995;270:13645–13651. doi: 10.1074/jbc.270.23.13645. [DOI] [PubMed] [Google Scholar]
- 96.Stenfeldt AL, Wenneras C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605–614. doi: 10.1111/j.1365-2567.2004.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matzinger P. Tolerance, danger, and the extended family. Annu.Rev.Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 98.Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir.Cell Mol.Biol. 1997;17:70–77. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- 99.Solomon A, Aloe L, Pe'er J, Frucht-Pery J, Bonini S, Bonini S, Levi-Schaffer F. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin.Immunol. 1998;102:454–460. doi: 10.1016/s0091-6749(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 100.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin.Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 101.Phipps S, Ying S, Wangoo A, Ong YE, Levi-Schaffer F, Kay AB. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–4612. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- 102.Plotz SG, Lentschat A, Behrendt H, Plotz W, Hamann L, Ring J, Rietschel ET, Flad HD, Ulmer AJ. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood. 2001;97:235–241. doi: 10.1182/blood.v97.1.235. [DOI] [PubMed] [Google Scholar]
- 103.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 104.Hirsch JG, Hirsch B. Paul Ehrlich and the discovery of the eosinophil. 1980:3–23. [Google Scholar]
- 105.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc.Natl.Acad.Sci.U.S.A. 1989;86:1348–1351. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin.Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp.Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc.Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 109.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin.Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 110.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 111.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin.Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Ghahary A, Moqbel R. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]