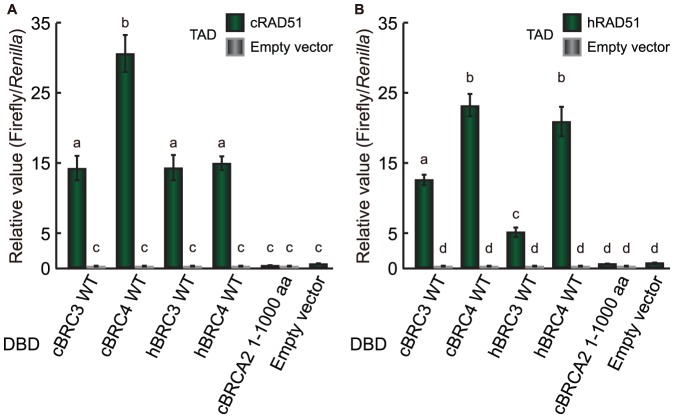
Figure 1. Interaction between cBRC3 and canine or human RAD51 detected by mammalian two-hybrid assay.
The level of the interaction between cBRC3 and cRAD51 was half of that observed with cBRC4 and similar to that with hBRC3 and hBRC4. The interaction between hRAD51 and cBRC3 was stronger than with hBRC3. (A) VP16 transactivation domain-fused cRAD51 construct or empty vector and a Gal4-DBD-fused cBRC3, cBRC4, hBRC3, hBRC4, or cBRCA2 N-terminus (1–1000 aa) were introduced into HeLa cells to determine their interaction with cRAD51 in a mammalian two-hybrid luciferase-based assay. HeLa cells were co-transfected with the BRC repeat constructs and cRAD51 expression vector constructs with the reporter plasmids pGluc and pRL-TK. The pRL-TK construct was used to normalize the transfection efficiency. Lysate luciferase activity was determined 48 h after transfection. (B) same as (A), except that VP16 transactivation domain-fused hRAD51 was used. The results are given as the mean (± standard error) (n = 3). Significance was examined by a one-way analysis of variance test, followed by a Tukey's post-test. Different letters indicate significant differences between the cells transfected with the indicated constructs. (p<0.01). DBD, DNA-binding domain; TAD, transactivation domain-fused proteins.