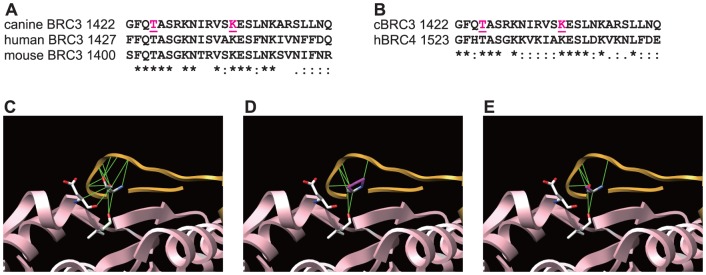
Figure 2. The T1425P mutation disrupted the conformation of canine BRC3 and RAD51 in silico.
(A) Amino acid sequence alignment of BRC3 among canines (Accession No. NP_001006654.2), humans (Accession No. NP_000050.2), and mice (Accession No. NP_033895.2). (B) Amino acid sequence alignment of cBRC3 and hBRC4. The pink characters indicate the positions of missense mutations in cBRC3. (C–E) The contacts between the residues of 1526T (C), T1526P (D), or T1526A (E) and hRAD51 or other residues of BRC4 were calculated and are depicted. Human BRC4 and hRAD51 are depicted by gold and pink ribbons, respectively. Solid green lines signify stable contacts as determined by the University of California, San Francisco Chimera software.