Abstract
A thiol-ene polymerization platform was used to synthesize peptide functionalized poly(ethylene glycol) (PEG) hydrogels, which were initially characterized and compared to theoretical predictions of Young’s modulus via a theoretical crosslinking density equation presented herein. After thorough characterization, this material system’s utility for answering specific biological hypotheses was demonstrated with the culture and observation of aortic valvular interstitial cells (VICs). Specifically, these materials were used to better understand the role of substrate elasticity and biochemical functionality on VIC α-smooth muscle (αSMA) expression and secretory properties (i.e., de novo ECM). The Young’s moduli of the hydrogels were varied from 28kPa (activating, 90% myofibroblasts) to 4kPa (non-activating, 15% myofibroblast) substrates, and the biochemical functionality was tailored by incorporating three small adhesive peptide sequences, RGDS, VGVAPG, and P15. To promote VIC adhesion, a basal [RGDS] of 0.8mM was used in all formulations, while the [VGVAPG] or [P15] were varied to be lower, equal, or higher than 0.8mM. The substrates with 1.2mM VGVAPG and all gels with P15 led to significantly higher αSMA expression for both stiff and soft substrates, as compared to 0.8mM RGDS alone. Importantly, all gel conditions were significantly lower than TCPS (~4–10 fold difference). The ECM produced significantly decreased as the total integrin binding peptide concentration increased, but was significantly higher than that expressed on TCPS. This easily tailored material system provides a useful culture platform to improve the fundamental understanding of VIC biology through isolating specific biological cues and observing VIC function.
Keywords: valvular interstitial cells, thiol-ene, hydrogels, integrin-binding peptides, ECM expression
1. Introduction
Valvular interstitial cells (VICs) are the main cell population in heart valves and are responsible for maintaining valve homeostasis [1]. In healthy valves, VICs typically reside in a quiescent fibroblast state, but can become activated into myofibroblasts upon injury to the valve [2]. In this activated state, they are known to be able to contract [1–5], proliferate [2,3,6], and express a host of different proteins including cytokines [2,5,6], matrix metalloproteinases (MMPs) [1,2,6,7], and extracellular matrix (ECM) molecules [2,3,6–9], all in an effort to maintain proper valve function. However, this delicate balance of VIC activation and secretory properties can also go awry if misregulation and repeated injuries occur. For example, the prolonged activation and elevated ECM expression of VICs can lead to calcific aortic stenosis [6,10–13].
The microenvironment of the valve has an acute influence on VIC phenotype and function, and researchers are only beginning to understand how valve attributes and conditions direct VIC behavior. Activation of VICs to the myofibroblast phenotype is known to depend on the culture substrate stiffness [2,6,7–9,14,15] and in response to specific proteins presented on culture substrates [1,9,11,12,16,17]. This increase in activation due to physical or biochemical cues has also been linked to subsequent increases in ECM production [1,17,18]. Understanding the complex interplay between cellular cues and matrix interactions, and how they influence VIC secretory properties, especially the deposition of ECM, would be extremely advantageous, when trying to design bioactive culture platforms to probe and eventually direct VIC function. To-date, few studies report on how VIC-material interactions influence the composition and deposition of ECM produced by VICs [12,17,19]. This is further complicated by the fact that when VICs are isolated from valve tissue and cultured on traditional tissue culture plasticware, essentially 100% of the VICs become activated myofibroblasts in less than 48 hours, which causes potentially non-physiologically relevant cellular responses [1].
To achieve precise control of VIC culture conditions, a thiol-ene step-growth photopolymerization was employed to fabricate a highly defined culture substrate. These materials were fabricated by reacting norbornene functionalized 8-armed PEG monomers with cysteine (i.e., thiol) functionalized small peptides in the presence of UV light and a photoinitiator. Through facile selection of the macromolecular precursors, the substrate stiffness can be manipulated by altering the molecular weight of poly(ethylene glycol) (PEG) monomers for large changes in gel modulus or performing the reaction off stoichiometry for small modulus adjustments [20–23]. The biochemical composition of the gel is also easily tailored to the hypothesis at hand, by simply introducing cysteine-containing peptides that are covalently bound within the gel network based on the initial reactant stoichiometry. By selecting integrin binding small peptides derived from larger ECM molecules to incorporate into the gels, one can study the effects of the cell-matrix relationship on VIC function and remove inherent confounding factors from the system, such as cytokine sequestering [3,9,24–29]. Additionally, the use of hydrophilic PEG as the base gel formulation creates a “blank slate” culture substrate to functionalize with specific proteins of interest, by minimizing non-specific protein adsorption [25–27]. The small peptide adhesion sequences, used herein, are derived from the larger ECM molecules found in the valve tissue; fibronectin (RGDS) [30], elastin (VGVAPG) [31], and collagen-1 (P15) [32].
Here, we endeavor to design VIC culture platforms utilizing the above described PEG based system to vary both substrate modulus and integrin binding events to gauge the importance of these two culture conditions on the activation and ECM expression of VICs.
2. Materials and Methods
2.1. Materials
Eight-armed poly(ethylene glycol) (MW: 20,000 and 40,000) was purchased from JenChem. All amino acids and resin for solid phase peptide synthesis (SPPS) were purchased from Anaspec and Novabiochem, respectively. Porcine hearts for VIC isolation were obtained from Hormel Inc. All other chemicals were purchased from Sigma-Aldrich, unless otherwise specified.
2.2. Synthesis of Eight-Armed PEG-Norbornene
Eight-armed PEG-norbornene (PEG-N, Figure 1a) (MW: 20,000 and 40,000) was synthesized as previously described by Fairbanks et al. [25] Briefly, the reaction was carried out under anhydrous conditions in the organic solvent dichloromethane (DCM), where a PEG solution was added drop-wise to a stirred solution of N,N′-dicyclohexylcarbodiimide (DCC) and norbornene acid, and allowed to react overnight at room temperature. The norbornene functionalized PEG in this solution was then precipitated in ice-cold ethyl ether, filtered, and re-dissolved in chloroform. This chloroform PEG solution was then washed with a glycine buffer and brine before being precipitated in ice-cold ethyl ether and filtered again. The filtered PEG was then placed in a vacuum chamber to remove excess ether. The percent functionalization of PEG arms with norbornene groups was determined using 1H-NMR and comparing the hydrogen peaks associated with the carbon adjacent to the ester linkage (~4.2ppm) to the hydrogen peaks associated with the PEG molecule (~3.6ppm). Supplemental Figure 7 demonstrates typical 1H-NMR spectra along with the integrated peak values. Only synthesis products with greater than 95% functionalization were used.
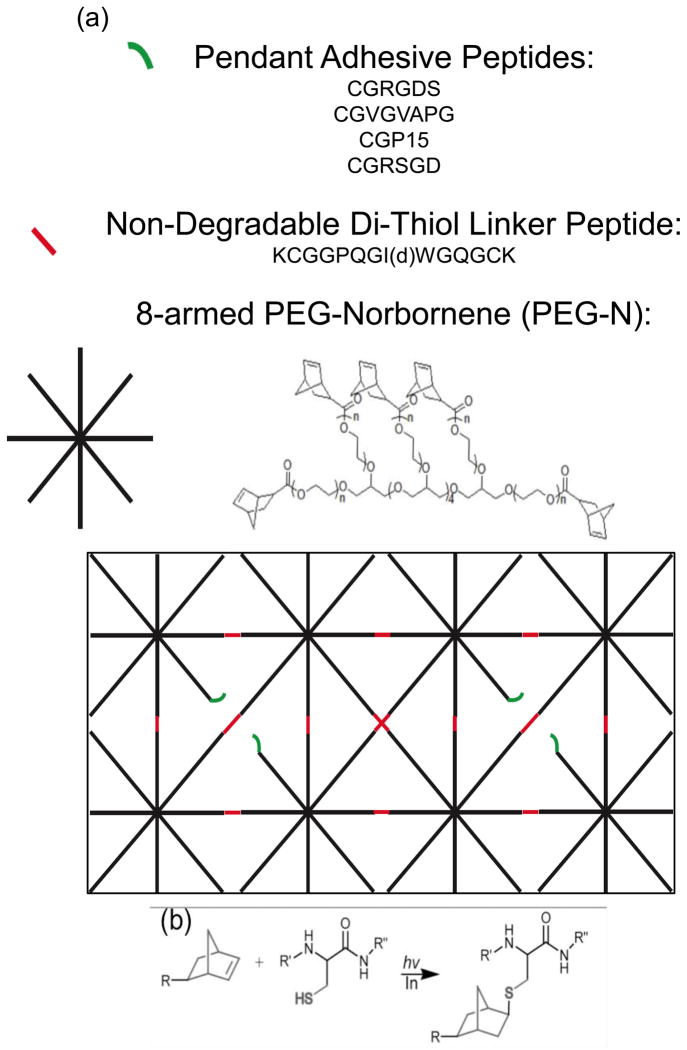
Figure 1.
(a) Monomers used for the fabrication of peptide functionalized PEG gels. The macromolecular precursors included; 8-armed PEG-N (where n=125 and 250 for 20 and 40kDa PEG, respectively), the non-degradable di-thiol linker peptide (where d denotes reversed chirality of the Ile), and the adhesive and scrambled pendant peptide sequences. These macromolecular monomers copolymerize to form a crosslinked network, as depicted with the idealized schematic of a relatively uniform mesh size. (b) Depiction of the reaction of a cysteine containing thiol with the norbornene functionality. R, R′, and R″ denote PEG, and the two adjacent amino acids in any of the peptide sequences, respectively.
2.3. Synthesis of Adhesive Small Peptide Sequences and Non-Degradable Di-Thiol Linker Peptide
Three peptides were chosen to include in the thiol-ene formulation, including one from fibronectin (CGRGDS), elastin (CGVGVAPG), and collagen-1 (CGGTPGPQGIAGQRGVV) (i.e., CGP15). A scrambled peptide (CGRSGD) was also synthesized to maintain the total pendant peptide concentration at 2mM. While 2mM of pendant peptide is added to the monomer solution, the final concentration available for interaction with the cells will be lower depending on incorporation efficiency and gel swelling. Supplemental Figure 1 illustrates that the actual surface concentration is approximately 25% of the initial 2mM (i.e., 500μM), and was not significantly different between gels of the two moduli used for VIC culture (i.e., 28 and 4 kPa). To avoid confusion, results are reported with the initial monomer solution peptide concentrations used to formulate the gels, as is typical in the literature. Additionally, an MMP cleavable di-cysteine (i.e., di-thiol) peptide derived from collagen-1 (KCGGPQGIWGQGCK) was also selected, based on previous success in 3D cultures [25, 33–36]. For the purpose of the present 2D cultures, the peptide linker was rendered non-degradable to MMPs by using the D conformation of isoleucine (I) instead of the naturally occurring L conformation (i.e., non-degradable di-thiol linker peptide). These peptides were synthesized using solid phase peptide synthesis (SPPS) on an Applied Biosystems model 433A or Tribute peptide synthesizer. After a 5wt% phenol trifluoroacetic acid (TFA) cleavage and ice cold ether precipitation, if the purity was found to be less than 95% via high performance liquid chromatography (HPLC), then large scale HPLC purification was performed. The correct eluate fraction, based on MW, was determined through matrix assisted laser desorption ionization (MALDI). The HPLC buffer was removed from the peptide in solution via lyophilization.
2.4. Fabrication and Characterization of PEG-Norbornene Gels
Eight-armed PEG-N (MW: 20,000 or 40,000), non-degradable peptide linker, photoinitiator hydroxyketone 2-hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone) (Irgacure 2959, I-2959, Ciba), and the cysteine-containing peptide CGRGDS were dissolved in phosphate buffered saline (PBS) to concentrations outlined in Table 1. These monomer solutions were injected between two sigmacoted glass slides separated by 1mm rubber spacers and placed under a 365nm UV light at 8.5mW/cm2 for 10 minutes to initiate the thiol-ene polymerization (Figure 1b). The resulting gels were allowed to swell overnight in PBS and their swollen mass and shear moduli were subsequently characterized. The gels were then lyophilized, and the dry polymer mass recorded. The equilibrium volumetric swelling ratio (Q) was calculated by using [28]
Table 1.
Macromolecular monomer compositions for the preparation of RGDS functionalized gels and used to study the range of moduli that are achieved by varying both the PEG monomer molecular weight and the functional group stoichiometry. PEG-N denotes PEG-Norbornene monomers and the molecular weights are given in kDa and nd linker peptide denotes the non-degradable di-thiol linker peptide. Off stoichiometry monomer compositions were achieved by decreasing the [nd linker peptide]3.
| PEG-N MW (kDa) | Thiol : Ene Ratio | [PEG-N] (mM)1 | [Ene] (mM)2 | [RGDS thiol] (mM) | [nd linker peptide] (mM) | [nd linker thiol] (mM) |
|---|---|---|---|---|---|---|
| 20 | 1:1 | 5.0 | 40.0 | 2.0 | 19.0 | 38.0 |
| 3:4 | 14.25 | 28.5 | ||||
| 2:3 | 12.65 | 25.3 | ||||
| 1:2 | 8.0 | 16.0 | ||||
| 40 | 1:1 | 2.5 | 20.0 | 2.0 | 9.0 | 18.0 |
| 3:4 | 6.75 | 13.0 | ||||
| 2:3 | 6.0 | 12.0 | ||||
| 1:2 | 4.5 | 9.0 |
The [Ene]=[PEG-N]*8 since the PEG monomer selected has 8 arms.
The [nd linker thiol]=[nd linker peptide]*2 as the peptide was synthesized with a cysteine amino acid (i.e., thiol side group) on either side of the sequence.
For on-stoichiometry reactions (1:1 thiols to enes), the sum of the [RGDS thiol] and [nd linker thiol] should equal the [Ene].
| (1) |
Where, Ms, Md, ρd, ρs, correspond to the swollen mass, the dry polymer mass, the polymer density, and the solvent density, respectively.
The rheometer used for shear modulus characterization was a parallel plate Ares that applied varying strains (γ) or frequencies (ω) from the bottom plate and the stress transducer plate above the gel measured the resulting displacement. Both strain and frequency sweeps were conducted to verify that the shear modulus measurements were taken in the linear viscoelastic regime. The strain and frequency that fell within this regime were 10% and 10 R/s, respectively.
2.5. Preparation of PEG-Norbornene Substrates for Cell Culture
In a sterile cell culture hood, eight-armed PEG-N (MW: 20,000), non-degradable crosslinking peptide, and 2mM of pendant mono-thiol peptides were dissolved in sterile PBS to yield a final monomer solution concentration of 5mM (i.e., 40mM [ene]), 19mM (i.e., 38mM [linker thiol]), and 2mM [mono-thiol peptide], respectively. Eight-armed PEG-N (MW: 40,000), non-degradable crosslinking peptide, and 2mM of pendant mono-thiol peptides were also dissolved in sterile PBS to yield a final monomer solution concentration of 2.5mM (i.e., 20mM [ene]), 6mM (i.e., 12mM [linker thiol]), and 2mM [mono-thiol peptide], respectively. To these monomer solutions, the photoinitiator I-2959 was also added, yielding a final concentration of 0.2mM. All solutions were sterile filtered using a 20μm porous filter before polymerization. The 2mM mono-thiol (i.e., pendant) peptide was comprised of 0.8mM RGDS and varying concentrations of VGVAPG or P15 were added (0.4, 0.8, and 1.2mM). A scrambled RSGD (0.8, 0.4, and 0mM) was used to maintain a total of 2mM pendant peptide, as the VGVAPG and P15 content was varied.
These monomer solutions were injected between two sterile, sigmacoted glass slides separated by sterile 1mm rubber spacers and placed under 365nm UV light at 8.5mW/cm2 for 10 minutes. The resulting gels were allowed to swell overnight in serum free media at 37°C before cell seeding.
2.6. VIC Isolation
Primary VICs were isolated from aortic leaflets, which were excised from fresh porcine hearts acquired from Hormel within 24 hours of slaughter via a sequential collagenase digestion as previously described by Filip et al. [4], and aliquots frozen until needed. Briefly, the leaflets were incubated in Earle’s balanced salt solution containing 250U/mL collagenase for 30 min to remove the endothelial lining, followed by a 60 min incubation in fresh collagenase solution to remove the VICs. The VIC suspension was filtered through a 100μm strainer to remove the degraded leaflets and centrifuged at 1000rpm for 10 minutes. The pellet was resuspended in Media 199 supplemented with 15v/v% fetal bovine serum (FBS), 2v/v% penicillin/streptomyocin, 0.4v/v% fungizone, and 0.2v/v% gentamicin (Invitrogen); plated on TCPS dishes; and cultured to 50% confluency at 37°C and 5% CO2. The VICs were then trypsinized, pelleted, and resuspended in 45v/v% M199 media, 50v/v% FBS, and 5v/v% dimethyl-sulfoxide (DMSO) to 1,000,000 cells/mL. This suspension was transferred to cryovials, which were then placed in a slow temperature gradient freezing box at −80°C overnight. These frozen vials were stored in a liquid nitrogen cooled tank until needed, and then thawed and expanded on tissue culture polystyrene (TCPS) in Media 199 supplemented with 15v/v% fetal bovine serum (FBS), 2v/v% penicillin/streptomyocin, 0.4v/v% fungizone, and 0.2v/v% gentamicin (Invitrogen) in an incubator set to 37°C and 5% CO2. Only VICs of the third passage were used for experiments.
2.7. Effect of Gel Stiffness and Integrin Binding on αSMA Expression
PEG-Norbornene gels were prepared as described above for cell culture. After a vial of frozen VICs were thawed, grown up to 50% confluency, and trypsinized, the pellet was resuspended to 1,000,000 cells/mL in 90μL of primary mammalian transfection buffer (Lonza) and added to 10μL of the same buffer containing 1μg/million cells of an alpha-smooth muscle actin promoter luciferase plasmid [1,14,37]. This solution was transferred to a transfection couvette, and the plasmid was added to the VIC cytoplasm via electric shock transfection. The transfected VIC solution was then transferred to 1mL of 15v/v% FBS M199 growth media and incubated for 15 minutes. The cell solution was then diluted with more 15% FBS media and seeded on top of the experimental gels at 20,000cells/cm2. After 24 hours the media was changed to 1% FBS media, to avoid significant proliferation and cell-cell contacts. The gels and VICs were then placed in an incubator at 37°C and 5% CO2. After 24 hours in 1% FBS media, the VICs were lysed using the glo-lysis buffer (promega) and frozen at −80°C for 2 hours. The lysates were then thawed and pelleted at 4°C to remove any cellular debris, and 100μL of the supernatant was transferred to a white 96-well plate. 50μL of the luciferase substrate (promega) was added to each sample and allowed to incubate at room temperature for 5 minutes. The luminescence of each sample was recorded as an indirect measure of the αSMA production, which is a hallmark indicator for a fibroblast that has been activated to the myofibroblast phenotype [5,9,10,14]. This luminescence was normalized to the amount of DNA present in the cell lysate by using a pico-green assay (invitrogen). To demonstrate that the soft 4kPa substrates were not selecting a senescent phenotype subpopulation of VICs, but that the VICs could still be activated to the secretory myofibroblast phenotype, 5ng/mL of soluble TGF-β1 was added to the culture media and αSMA expression assessed. αSMA expression was significantly up-regulated compared to soft substrates without TGF-β1 treatment, regardless of peptide condition identity (Supplemental Figure 4). This result would seem to indicate that the soft substrates are not selecting for a senescent subpopulation of the seeded VICs, but they are still able to undergo activation to the myofibroblast phenotype.
2.8. Effect of Gel Stiffness and Integrin Binding on VIC ECM Secretion
The effects of substrate stiffness and integrin binding events on VIC ECM production were investigated through the incorporation of [3H]glycine into de novo glycosaminoglycans (GAGs) and other glycoproteins, elastin, and collagen through a sequential enzyme digestion, termed the TEC (trypsin, elastase, and collagenase) assay [12,19,38,39]. Standard literature protocols were followed.
PEG-N gels were prepared, as described above for cell culture. After VICs were grown up to 50% confluency, they were trypsinized, pelleted, resuspended in 1% serum media containing 1μCi/mL [3H]glycine, and seeded on the experimental gels in ultra-low adhesion culture plates (Corning) at a density of 20,000 cells/cm2. The supplemented media was changed and saved every 3 days for 2 weeks. The same procedure was followed for cultures intended for cell counting (i.e., normalization), except that the media was supplemented with the same volume of carrier solution, minus the [3H]glycine. At day 3 and 14, the cells in the non-radioactive samples were trypsinized and counted via a hemacytometer. The saved radioactive media was transferred to a treated TCPS plate and incubated at 37°C overnight to ensure equilibrium adsorption of the ECM molecules in solution. The reader should note that there might be some variability in the adsorption of each ECM molecule secreted into the media onto the TCPS. However, given that all of the ECM molecules are comprised of proteins with stabilizing hydrophobic interactions, it is assumed that all of the ECM molecules will have a high affinity of binding to the TCPS. The experimental gels were transferred to PBS and incubated overnight at 37°C to remove any non-incorporated [3H]glycine swollen into the gel. Both the gels and the saved media plates were subjected to sequential digestion and incubation with trypsin, elastase, and collagenase as described in reference 38. Each enzyme degradation sample represents the fraction of produced tissue that is susceptible to proteolytic cleavage by each respective enzyme (i.e., non-specific cleavage may occur). However, Supplemental Figure 5 demonstrates that each enzyme degradation fraction contains mostly the desired ECM, with only small amounts of undesired ECM (i.e., ~10% error). With this issue in mind and to ease reading of this manuscript, each degradation fraction is referred to as the ECM molecule that comprises the majority of the sample, which is approximately 90% of the total amount. Radioactivity in samples from each gel and degradation step was measured by scintigraphy (Packard Tri-Carb 2300TR).
2.9. Statistics
Data are presented as mean ± standard error. For rheological, equilibrium swelling ratio, and ECM expression measurements, n=6, and for VIC αSMA expression, n=3. Data was compared using a student’s t-test and an ANOVA (for ECM compositional results found in Figure 5) using the software JMP 9.0, and significance was established for p≤0.05.
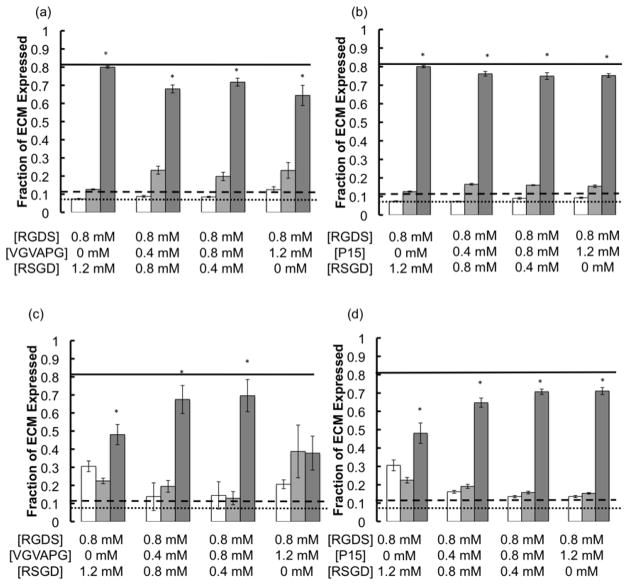
Figure 5.
The composition of ECM (Trypsin susceptible/GAGs and glycoproteins (white bars), elastase susceptible/elastin (light gray bars), and collagenase susceptible/collagen (dark gray bars) being produced as a function of integrin binding peptide identity (VGVAPG (a & c) and P15 (b & d)) and concentration on both 28kPa stiff (a & b) and 4kPa soft (c & d) gels. The solid line represents collagen expression, the dashed line represents elastin expression, and the dotted line represents GAG/glycoprotein expression on uncoated TCPS. VICs were seeded at a density of 20,000 cells/cm2 in 1% serum media and ECM composition deposited was measured on day 14. On the stiff substrates, collagen was significantly more expressed than elastin or GAGs/glycoproteins. On softer substrates, the composition of the ECM being expressed was still mostly collagen, with the exception of the 0.8mM RGDS + 1.2mM VGVAPG condition, which had fractions of collagen and elastin that were no longer significantly different being produced. (* p≤0.05 as compared to GAGs/glycoproteins and elastin fractions for the same stiffness and peptide composition condition).
3. Results
3.1. Characterizing and Tailoring the Thiol-Ene Substrate
Gels with a wide range of moduli were synthesized with the monomer compositions outlined in Table 1, using the monomers depicted in Figure 1, and their resulting shear moduli (G) were quantified using parallel plate rheometry. These experimentally measured shear modulus values were converted to Young’s Modulus (E) using rubber elasticity theory,
| (2) |
Here, ν is Poisson’s ratio, which was assumed to be 0.5 for an incompressible material and then plotted with the theoretically calculated Young’s Modulus (Etheo) values, using equations 3–5, as reported in Figure 2.
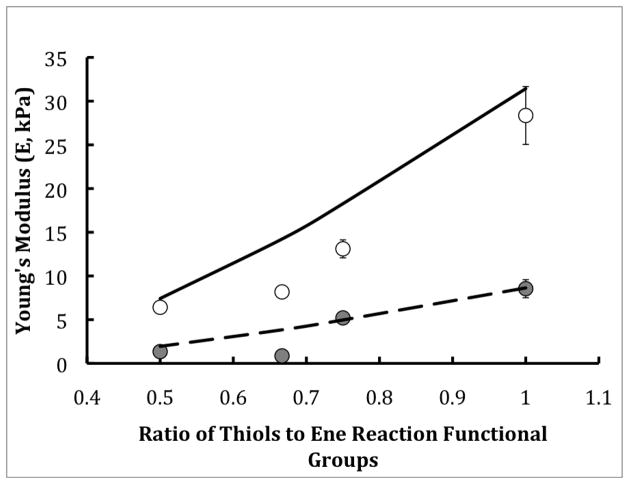
Figure 2.
Theoretical (lines) and experimental (points) Young’s Modulus as a function of the thiol to ene functionality group ratio for two different PEG monomer molecular weights 20kDa (open circles and solid line) and 40kDa (filled cirlces and dashed line). All gels were swollen to equilibrium before measurements. A wide range of moduli is achieved by simple variations in the monomer composition.
To calculate Etheo, first the theoretical equilibrium swelling ratio (QTheo) was calculated using [40]
| (3) |
Where, ν is the volume of one monomer unit (i.e., ν =(1−2*χ)*b3); b is the Kuhn length of one PEG unit, χ is the solvent-polymer interaction parameter; N is the number of PEG repeat units between crosslinks, and φo is the initial volume fraction of polymer. This equation is valid for systems where 1/Q<φ**<φo (intermediate solvent regime), where φ** ≈1/√N (i.e., the critical concentration of polymer) [40].
The theoretical crosslinking density (ρx,theo) was then calculated using
| (4) |
Here, f is the number of PEG monomer arms (e.g, 8 for an 8-arm PEG) and Qo is the initial swelling ratio. Finally, the theoretical Young’s modulus (Etheo) was calculated using rubber elasticity theory [20]
| (5) |
Where ν is Poisson’s ratio and assumed to be 0.5 for an incompressible material, R is the ideal gas constant, and T is absolute temperature.
Figure 2 demonstrates that when the MW of the PEG monomer was decreased by a factor of 2 (i.e., from 40kDa to 20kDa), the resulting moduli was increased by a factor of 3, which was a statistically significant increase. Additionally, by decreasing the ratio of thiol to ene functional groups, the modulus for each PEG MW was significantly decreased, but to a lower extent than by altering the monomer MW. By using both of these methods of monomer composition manipulation, one is able to obtain a wide range of moduli (i.e., 1–28 kPa) to prepare cell culture substrates. These exact formulations were used for all of the following cell culture experiments.
From this wide range of accessible moduli, two monomer compositions were selected to yield one activating and one non-activating cell culture substrate. The measured shear modulus values (G) and swelling ratios (Q) of the selected monomer compositions (i.e., activating and non-activating substrates) are summarized in Table 2, along with the theoretical and experimental crosslinking densities (ρx) calculated using equation 4 and rubber elasticity theory [28].
Table 2.
Experimentally measured and theoretically calculated material properties of thiol-enePEG hydrogels prepared for VIC culture. G is the equilibrium swollen shear modulus; Q is the equilibrium volume swelling ratio; ρx is the network crosslinking density; and E is the equilibrium swollen Young’s modulus. This data demonstrates that the gels formed with the 20kDa and 40kDa PEG precursors yield modulus values very similar to those theoretically predicted.
| In Text Descriptions | GExp (kPa) | GTheo (kPa) | QExp | QTheo | ρx Exp. (mM) | ρx Theo (mM) | EExp (kPa) | ETheo (kPa) | |
|---|---|---|---|---|---|---|---|---|---|
| 20kDa PEG-N | Stiff | 9.5 | 14 | 13.4 | 13.7 | 7.3 | 10.2 | 28.4 | 31.4 |
| 40kDa PEG-N | N/A | 2.8 | 4 | 19.4 | 20.3 | 3.1 | 3.2 | 8.6 | 8.6 |
| 40kDa PEG-N (2:3, thiols : enes) | Soft | 1.2 | 2 | 32.3 | 23.2 | 1.4 | 1.5 | 3.6 | 3.9 |
| (6) |
Gels formed with the 20kDa PEG and reacted at 1:1 stoichiometry yielded a Young’s modulus of 28kPa, which induced VIC activation to the myofibroblastic phenotype for 80% of the population (Supplemental Figure 2). This monomer formulation was selected as the stiff, activating culture substrate in all subsequent studies. The 40kDa PEG reacted at 1:1 stoichiometry hydrogels yielded a shear modulus of 9kPa, which caused VIC activation that was not significantly lower than the 20kDa PEG gels (Supplemental Figure 2). To lower the modulus of the 40kDa PEG gels, the stoichiometry of the thiol:ene functionalities was reduced from 1:1 to 2:3. Step-growth polymerizations form relatively ideal networks when reacting functionality concentrations are equal [20]. However, gelation still occurs if the reactive functional concentration is minimally shifted (such that the average functional group concentration (i.e., the average number of functional groups per monomer molecule for both monomers in the mixture [41]) remains greater than 2), rendering the resulting polymer networks less crosslinked and leading to moderate decreases in the bulk gel modulus. This shift in the reactive functional group stoichiometry removes a third of the potential crosslinks throughout the bulk of the gel, which correspondingly reduces the bulk gel modulus by a third, according to rubber elasticity theory [28]. The shear modulus of these off-stoichiometry 40kDa (2:3) gels was reduced to 4kPa, which also significantly reduced VIC activation to approximately 15%, as compared to the 20kDa gels (Supplemental Figure 2). Thus, the 2:3 off-stoichiometry 40kDa PEG monomer formulation was used as the soft and non-activating culture substrate in all subsequent studies.
3.2. Effects of Integrin Binding & Substrate Stiffness on α-Smooth Muscle Actin Expression
To quantify αSMA expression, VICs were transfected with an αSMA promoter luciferase plasmid, seeded on experimental gels, lysed after two days, and the luminescence measured with a luciferase substrate and normalized to total mass of DNA. Because this assay inherently does not distinguish between diffuse and polymerized αSMA, immunostained images of αSMA organization were compared to both luminescence values and a western blot of fibrous αSMA on both stiff and soft substrates in Supplemental Figure 3. These images demonstrate the difference in αSMA organization (i.e., myofibroblast activation), even though the luminescence for the soft substrate is not substantially lower than that of the stiff gels, yet is significantly reduced. Moving forward with this result in mind, the luminescence assay allows a more quantitative and sensitive measurement of αSMA expression. Here, three small peptides were used to remove any confounding effects of whole ECM molecules.
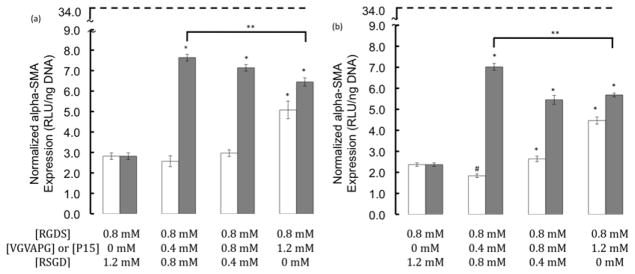
Concentrations and combinations of three different small integrin-binding peptides, RGDS, VGVAPG, and P15, were varied, and the effect of epitope composition on αSMA expression was measured (Figure 3). The condition with the highest concentration of VGVAPG had significantly increased luminescence (i.e., αSMA expression) as compared to all other conditions for both the stiff and soft substrates. This result demonstrates that a culture substrate with a higher amount of VGVAPG, as compared to RGDS, can cause a marked increase in αSMA expression, which agrees with previous whole ECM activation studies [16]. When RGDS was combined with the collagen-1 derived P15 peptide at any ratio, the αSMA expression was significantly higher than the 0.8mM RGDS control for both stiff and soft substrates. Additionally, the level of αSMA expression on both of the two substrate stiffnesses was an order of magnitude lower (p<0.01) than that observed on uncoated TCPS (dashed line).
Figure 3.
Normalized αSMA expression (RLU/ng DNA) as a function of integrin binding peptide identity (VGVAPG, white bars and P15, gray bars) and concentration. The dashed line represents expression levels on uncoated TCPS. Elasticity effects were also examined using (a) 28kPa stiff gels and (b) 4kPa soft gels. VIC were cultured for 2 days in 1% serum media at a density of 20,000 cells/cm2. (* and # p≤0.05 higher or lower than the 0.8mM RGDS control, respectively. ** p<0.05 lower as compared to the 0.4mM P15 condition).
3.3. Effects of Integrin Binding and Substrate Stiffness on VIC ECM Expression
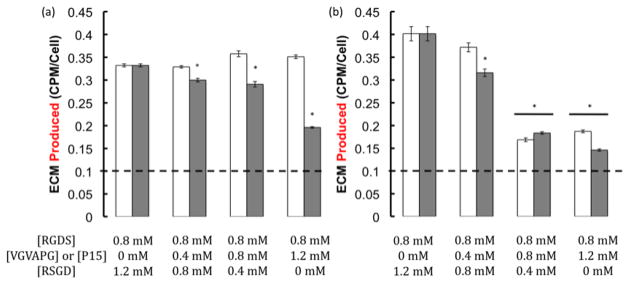
To understand how biophysical and biochemical cues might be used to direct de novo tissue deposition of VICs, activating and non-activating substrates were again used in conjunction with three different integrin binding small peptides, RGDS, VGVAPG, and P15, and the ECM produced, as well as its composition, were assessed using the TEC assay after 14 days in culture [12,19,38,39]. Figure 4 shows the differences in ECM production for each peptide and stiffness condition. On the stiff substrate, when P15 was combined with RGDS, the amount of ECM being produced decreased as the total available integrin binding peptide concentration increased. A similar result was observed for both VGVAPG and P15 on the soft substrates. All conditions were significantly higher than the amount expressed on uncoated TCPS (dashed line).
Figure 4.
ECM produced per cell as a function of integrin binding peptide identity (VGVAPG, white bars and P15, gray bars) and concentration for gels of two different stiffnesses (28kPa, (a) and 4kPa (b)). The dashed line represents expression levels on uncoated TCPS. VICs were seeded at a density of 20,000 cells/cm2 in 1% serum media and ECM production was measured on day 14. ECM produced on both the stiff and soft substrates decreased with increasing total available integrin binding peptide concentration. (* p≤ 0.05 as compared to 0.8mM RGDS control).
Figure 5 demonstrates the differences in composition of the expressed ECM. The stiff and soft substrates generally selected for collagen expression over elastin or GAGs/glycoproteins, regardless of peptide identity, with percentages being ~70%, ~30%, ~20%, respectively. However, the soft plus 1.2mM VGVAPG caused elastin to be expressed at a level not statistically different than that of collagen (Figure 5c). Interestingly, the fraction of collagen expression was significantly reduced from that on TCPS (solid line) for all of the soft substrate conditions and the stiff experimental conditions (i.e., not control).
4. Discussion
Thiol-ene photopolymerization has recently been established as a robust route for the synthesis of peptide functionalized PEG hydrogels [25]. In addition, Benton et al. recently demonstrated the usefulness of this gel system for the three-dimensional culture of primary porcine VICs [42]. Here, the ability to control the biochemical functionality (i.e., small peptide sequence incorporated) independently from the biomechanical properties or gel modulus via PEG monomer MW selection was exploited to better understand how these parameters influence αSMA and ECM expression properties of VICs. Towards this goal, two eight-armed PEG monomer molecular weights were chosen, 20 and 40kDa, to vary the substrate modulus from 9 to 28 kPa. As the PEG molecular weight is increased, the distance between crosslinks is effectively increased, which correspondingly decreases the bulk gel modulus, according to rubber elasticity theory [28]. Because of the direct correlation between crosslinking density and resulting gel modulus, reacting the step-growth thiol-ene networks off stoichiometry (i.e., removing potential crosslinks) causes corresponding decreases in the resulting gel modulus. For example, if the monomers were reacted at a ratio of 1:2 thiol to ene functionalities, then the predicted modulus is only one half of a gel formed from a mixture that is reacted at 1:1 stoichiometry. This characteristic of the step-growth networks allows for facile manipulation of the bulk gel physical properties.
Experimental modulus values for the higher molecular weight 40kDa monomer condition showed good agreement with the theoretically predicted values, with only minor deviations. However, the lower MW 20kDa PEG monomer conditions were consistently lower than the theoretically predicted values. This discrepancy may be attributed, in part, to the shorter PEG arms that may lead to a reduced local reaction volume and increased probability of reacting back with an arm on the same PEG molecule, forming loops and/or dangling ends (i.e., non-idealities) within the network structure. These loops and dangling ends remove potential crosslinks within the network structure (i.e., 2 and 1, respectively), which ultimately decreases the bulk modulus achieved. Regardless, the ability to adeptly control the bulk substrate modulus through controlled variations in the initial monomer formulations is extremely important when trying to shift the phenotype of VICs to an activated myofibroblast or render them to the native quiescent fibroblast state [2,6,7–9,14,15]. Small variations in the level of VIC activation can have a significant influence on VIC behavior and function, such as contractility [1,10] and ECM expression [1,17,18].
Previous studies have demonstrated that both the substrate stiffness [14] and adhesive protein [1,9,11,12,16,17] can influence the activation of VICs. However, most of the latter studies were completed on tissue culture polystyrene (TCPS) coated with whole ECM molecules individually. There is growing recognition, however, that while the TCPS culture platform allows for facile experiment implementation, the extremely stiff modulus causes cells to respond in a potentially non-physiologically relevant manner [43]. To address this issue, several alternative culture platforms, both natural [3,12,19], and synthetic [7,10,14,23,25,27,33–37,42], have been developed. Here, the facile manipulation and tailorability of PEG hydrogels has been demonstrated. The chemistry used to form these hydrogels also allows for user-defined selection of biochemical cues covalently linked within the gel network [25]. While whole ECM molecules may be entrapped within the network structure, small peptides are a very attractive alternative to alleviate potential confounding factors associated with whole ECM molecules, such as cytokine sequestration [5]. These small peptides can span a wide range of properties, from integrin binding [30–32] to specific cytokine affinity binding sequences [44,45]. Here, sequences from fibronectin (RGDS), elastin (VGVAPG), and collagen-1 (P15) were used in conjunction with activating (stiff, 28kPa) and non-activating (soft, 4kPa) substrates. The baseline of 0.8mM RGDS caused moderate αSMA expression, with organization into fibers only being seen on the stiff substrates. The combination of RGDS and 1.2mM elastin derived VGVAPG caused significantly increased αSMA expression over the control. This result is in agreement with whole ECM coated TCPS work [16], where elastin caused significantly more activated VICs than the uncoated control. Interestingly, the combinations of RGDS and P15 were all significantly higher than the control, regardless of substrate stiffness. To our knowledge, no study has included both fibronectin and collagen-1 coated surfaces to observe changes in VIC activation or αSMA expression. Additionally, as the concentration of P15 was increased, αSMA expression was significantly decreased, which also agrees well with the activation reducing potential of collagen-1 on TCPS [16]. The dramatically increased activation of RGDS combined with P15 over the 0.8mM RGDS control could suggest that the combination of these two integrin binding events causes a pathological intracellular signaling cascade, only present as collagen expression pervades the entire leaflet during the onset of aortic stenosis.
It is also important to note that the levels of αSMA expression on all gel conditions were an order of magnitude lower than the levels observed on uncoated TCPS (p≤0.01). This result demonstrates the non-physiologically relevant level of activation caused by the GPa stiffness of the TCPS, and this super-activated VIC population probably does not respond to biological stimuli as they might in vivo.
The sensitivity of VICs to the slight differences in mechanical and biochemical activation is extremely evident in not only the αSMA expression, but also in the composition of ECM produced. When VICs are activated to the myofibroblast phenotype, this phenotypic change appears to be correlated with the fraction of collagen being secreted, as the fractions of collagen on the soft substrates are significantly lower than those found on the stiff (as determined via an ANOVA statistical analysis). This result agrees with disease progression in vivo [6,46], which is attributed to collagen-1 pervading throughout the entire valve leaflet, causing impaired valve flexibility and function. It appears that only the VICs on soft substrates are able to produce levels of other ECM molecules, like elastin, that are no longer significantly different from the collagen fraction. This result suggests that gels (i.e., culture substrate stiffnesses closer to that of the native microenvironment) may reduce the percentage of collagen being expressed and allow for other matrix molecules, like elastin, found in healthy, functioning valves to be expressed. This knowledge may prove extremely important for the directed regeneration of aortic valve tissue for transplantation treatments.
It is also interesting to note that the levels of collagen expression on all of the gel formulations were lower than that found on uncoated TCPS. This result suggests that culture of VICs on TCPS may lead to very different biological observations, and ultimately promote a disease-like phenotype and behavior, limiting its utility as a platform for studying and understanding healthy VIC biology.
Interestingly, as the total available integrin-binding peptide concentration was increased (not the total [pendant peptide], as this is held constant at 2mM), the amount of ECM produced significantly decreased for both the stiff and soft culture substrates. This result suggests that as the amount of cell-matrix interactions are increased, VICs are directed to express less de novo ECM, which could have major implications in valve tissue regeneration. Additionally, it is important to note that the less activated VICs (i.e., cultured on gels) are able to express significantly higher amounts of ECM than the highly activated VICs on TCPS. This result may also be related to the amount of cell-matrix interactions the VICs are experiencing on the TCPS. Collectively, these studies suggest the need for a deeper understanding of how microenvironmental signals could be used to direct increased amounts of de novo tissue deposition, while retaining healthy valve compositional ECM control.
5. Conclusions
VIC αSMA and ECM expression were studied on culture substrates of varying stiffnesses and adhesive peptide combinations and concentrations. VICs significantly up-regulated αSMA expression when cultured on gels with 1.2mM VGVAPG and for all formulations with P15, regardless of substrate stiffness, when compared to the 0.8mM RGDS control. Most importantly, all gel conditions were an order of magnitude lower than the uncoated TCPS, which suggests that gel platforms could allow for a much more physiologically relevant microenvironment for VIC expansion and characterization. ECM produced decreased as total available integrin-binding peptide concentration was increased, and all gel conditions were significantly higher than TCPS. The ECM composition was mostly collagen, regardless of peptide identity, on both substrate stiffnesses. However, the fraction of collagen expression on the gels was significantly lower than that on the TCPS. Collectively, these results suggest that both the substrate stiffness and integrin binding events may play an important role in directing the level of VIC activation, as well as the amount and composition of ECM produced by VICs. This work also demonstrates that the thiol-ene PEG based system is easily tailorable in both modulus and biochemical functionalization, and provides a useful cell culture system to introduce selected cues to answer specific biological questions.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Chris Bowman for use of the rheometer and theoretical crosslinking density discussion, and Dr. Nancy Rice and Dr. Leslie Leinwand for supplying the α-smooth muscle actin promoter luciferase plasmid and radioactive certified lab space. The authors would also like to acknowledge funding from HHMI and NIH (R01 HL089260). Technical discussions with Dr. April Kloxin, Dr. Peter Mariner, and Mr. Troy Gould are also greatly appreciated.
Abbreviation Glossary
- αSMA
alpha-smooth muscle actin
- ECM
extracellular matrix
- GAGs
glycosaminoglycans
- MMPs
matrixmetalloproteinases
- MW
molecular weight
- nd-linker peptide
non-degradable linker (i.e., dithiol) peptide
- PEG
poly(ethylene glycol)
- PEG-N
norbornene functionalized poly(ethylene glycol)
- RLU
relative light units
- TCPS
tissue culture poly(styrene)
- TEC Assay
Trypsin, elastase, collagenase assay
- TGF-β1
transforming growth factor-β1
- VICs
valvular interstitial cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker GA, Masters KS, Shah DN, Anseth KA, Leinwand LA. Valvular Myofibroblast Activation by Transforming Growth Factor-Beta: Implications for Pathological Extracellular Matrix Remodeling in Heart Valve Disease. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 2.Chester AH, Taylor PM. Molecular and Functional Characteristics of Heart-Valve Interstitial Cells. Phil Trans R Soc B. 2007;362:1437–1443. doi: 10.1098/rstb.2007.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cushing MC, Jaeggli MP, Masters KS, Leinwand LA, Anseth KA. Serum Deprivation Improves Seeding and Repopulation of Acellular Matrices with Valvular Interstitial Cells. Journal of Biomedical Materials Research Part A. 2005;75A(1):232–241. doi: 10.1002/jbm.a.30412. [DOI] [PubMed] [Google Scholar]
- 4.Filip DA, Radu A, Simionescu M. Interstitial Cells of the Heart Valves Possess Characteristics Similar to Smooth Muscle Cells. Circ Res. 1986;59:310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- 5.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast Contraction Activates Latent TGF-β1 From the Extracellular Matrix. Journal of Cell Biology. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chester AH, Latif N, Yacoub MH, Taylor PM. Vascular Complications in Human Disease, Chapter 18: Aortic Valve: From Function to Tissue Engineering. London, England: Springer London; 2008. pp. 229–238. [Google Scholar]
- 7.Adelow C, Segura T, Hubbell JA, Frey P. The Effect of Enzymatically Degradable Poly(Ethylene Glycol) Hydrogels on Smooth Muscle Cell Phenotype. Biomaterials. 2008;29:314–326. doi: 10.1016/j.biomaterials.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Long JL, Tranquillo RT. Elastic Fiber Production in Cardiovascular Tissue-Equivalents. Matrix Biology. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 9.Hinz B. Formation and Function of the Myofibroblast During Tissue Repair. Journal of Investigation Dermatology. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 10.Benton JA, Kern HB, Anseth KS. Substrate Properties Influence Calcification in Valvular Interstitial Cell Culture. J Heart Valve Dis. 2008;17(6):689–699. [PMC free article] [PubMed] [Google Scholar]
- 11.Gu X, Masters K. Regulation of Valvular Interstitial Cell Calcification by Adhesive Peptide Sequences. Journal of Biomedical Materials Research Part A. 2010;93A(4):1620–1630. doi: 10.1002/jbm.a.32660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked Hyaluronan Scaffolds as a Biologically Active Carrier for Valvular Interstitial Cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez KJ, Masters KS. Regulation of Valvular Interstitial Cell Calcification by Components of the Extracellular Matrix. J Biomed Mater Res A. 2008;90(4):1043–1053. doi: 10.1002/jbm.a.32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloxin AM, Benton JA, Anseth KS. In situ Elasticity Modulation with Dynamic Substrates to Direct Cell Phenotype. Biomaterials. 2009;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinz B, Gabbiani G. Mechanisms of Force Generation and Transmission by Myofibroblasts. Current Opinion in Biotechnology. 2003;14(5):538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Cushing MC, Liao JT, Anseth KS. Activation of Valvular Interstitial Cells is Mediated by Transforming Growth Factor-β1 Interactions with Matrix Molecules. Matrix Biology. 2005;24:428–437. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Cushing MC, Liao JT, Jaeggli MP, Anseth KS. Material-based Regulation of the Myofibroblast Phenotype. Biomaterials. 2007;28:3378–3387. doi: 10.1016/j.biomaterials.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Mann BK, Schmedlen RH, West JL. Tethered-TGFβ1 Increases Extracellular Matrix Production of Vascular Smooth Muscle Cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 19.Masters KS, Shah DN, Walker G, Leinwand LA, Anseth KS. Designing Scaffolds for Valvular Interstitial Cells: Cell Adhesion and Function on Naturally Derived Materials. J Biomed Mater Res A. 2004;71(1):172–180. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 20.Bowman CN, Kloxin CJ. Toward and Enhanced Understanding and Implementation of Photopolymerization Reactions. AIChE Journal. 2008;54(11):2775–2795. [Google Scholar]
- 21.Hoyle CE, Bowman CN. Thiol–Ene Click Chemistry. Angew Chem Int Ed. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 22.Hoyle CE, Lee TY, Roper T. Thiol–Enes: Chemistry of the Past with Promise for the Future. Journal of Polymer Science: Part A: Polymer Chemistry. 2004;42:5301–5338. [Google Scholar]
- 23.Hossainy SFA, Hubbell JA. Molecular Weight Dependence of Calcification of Polyethylene Glycol Hydrogels. Biomaterials. 1994;15(11):921–925. doi: 10.1016/0142-9612(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Shah DN, Recktenwall-Work SM, Anseth KS. The Effect of Bioactive Hydrogels on the Secretion of Extracellular Matrix Molecules by Valvular Interstitial Cells. Biomaterials. 2008;29:2060–2072. doi: 10.1016/j.biomaterials.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A Versatile Synthetic Extracellular Matrix Mimic Via Thiol-ene Photopolymerization. Advance Materials. 2009;21(48):5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hern DL, Hubbell JA. Incorporation of Adhesion Peptides into Nonadhesive Hydrogels Useful for Tissue Resurfacing. Journal of Biomedical Materials Research Part A. 1997;39(2):266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, Davies N. The Selective Modulation of Endothelial Cell Mobility on RGD Peptide Containing Surfaces by YIGSR Peptides. Biomaterials. 2005;26:167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Bryant SJ, Anseth KS. Scaffolding in Tissue Engineering, chapter Photopolymerization of Hydrogel Scaffolds. Marcel Dekker, Inc; 2005. [Google Scholar]
- 29.Cellesi F, Tirelli N, Hubbell JA. Towards a Fully-Synthetic Substitute o Alginate: Development of a New Process Using Thermal Gelation and Chemical Cross-linking. Biomaterials. 2004;25:5115–5124. doi: 10.1016/j.biomaterials.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Pierschbacher MD, Ruoslahti E. Cell Attachment Activity of Fibronectin can be Duplicated by Small Synthetic Fragments of the Molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 31.Gobin AS, West JL. Val-ala-pro-gly, an Elastin-derived Non-integrin Ligand: Smooth Muscle Cell Adhesion and Specificity. Journal of Biomed Materials Res A. 2003;67(1):255–259. doi: 10.1002/jbm.a.10110. [DOI] [PubMed] [Google Scholar]
- 32.Hennessy KM. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30:1898–1909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raeber GP, Lutolf MP, Hubbell JA. Mechanisms of 3-D Migration and Matrix Remodeling of Fibroblasts within Artificial ECMs. Acta Biomaterialia. 2007;3:615–629. doi: 10.1016/j.actbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Lutolf MP, Hubbell JA. Synthesis and Physicochemical Characterization of End-linked Poly(ethylene glycol)-co-peptide Hydrogels Formed by Michael-Type Addition. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 35.Raeber GP, Lutolf MP, Hubbell JA. Molecularly Engineered PEG Hydrogels: A Novel Model System for Proteolytically Mediated Cell Migration. Biophysical Journal. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson J, Hubbell JA. Enhanced Proteolytic Degradation of Molecularly Engineered PEG Hydrogels in Response to MMP-1 and MMP-2. Biomaterials. 2010;31(30):7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 37.Benton JA, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins Block Calcific Nodule Formation of Valvular Interstitial Cells by Inhibiting α-Smooth Muscle Actin Expression. ATVB. 2009;29:1950–1957. doi: 10.1161/ATVBAHA.109.195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott-Burden T, Resink T, Burgin M, Buhler F. Extracellular matrix: Differential Influence on Growth and Biosynthesis Patterns of Vascular Smooth Muscle Cells from SHR and WKY rats. J Cell Physiol. 1989;141:267–274. doi: 10.1002/jcp.1041410206. [DOI] [PubMed] [Google Scholar]
- 39.Mann BK, Tsai AT, Scott-Burden T, West JL. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20(23–24):2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 40.Rubinstein Michael, Colby Ralph H. Polymer Physics. Great Clarendo St, Oxford NY: Oxford University Press; 2003. 7.4.3 Swelling in Good Solvents; pp. 278–279. Print. [Google Scholar]
- 41.Odian George. Principles of Polymerization. Hoboken, NJ: John Wiley & Sons, Inc; 2004. Step Polymerization-Crosslinking; p. 105. Print. [Google Scholar]
- 42.Benton JA, Fairbanks BD, Anseth KS. Characterization of Valvular Interstitial Cell Function in Three Dimensional Matrix Metalloproteinase Degradable PEG Hydrogels. Biomaterials. 2009;30(34):6593–6603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):645–647. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 44.Young GD, Murphy-Ullrich JE. The Tryptophan-rich Motifs of the Thrombospondin Type 1 Repeats Bind VLAL Motifs in the Latent Transforming Growth Factor-β Complex. J of Bio Chem. 2004;279(46):47633–47642. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]
- 45.Dotor J, Lopez-Vazquez AB, Lasarte JJ, Sarobe P, Garcia-Granero M, Riezu-Boj JI, Martinez A, Feifoo E, Lopez-Sagaseta J, Hermida J, Prieto J, Borras-Cuesta F. Identification of peptide inhibitors of transforming growth factor beta 1 using a phage-displayed peptide library. Cytokine. 2007;39:106–115. doi: 10.1016/j.cyto.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Liu AC, Joag VR, Gotlieb AI. The Emerging Role of Valve Interstitial Cell Phenotypes in Regulating Heart Valve Pathology. The American Journal of Pathology. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.