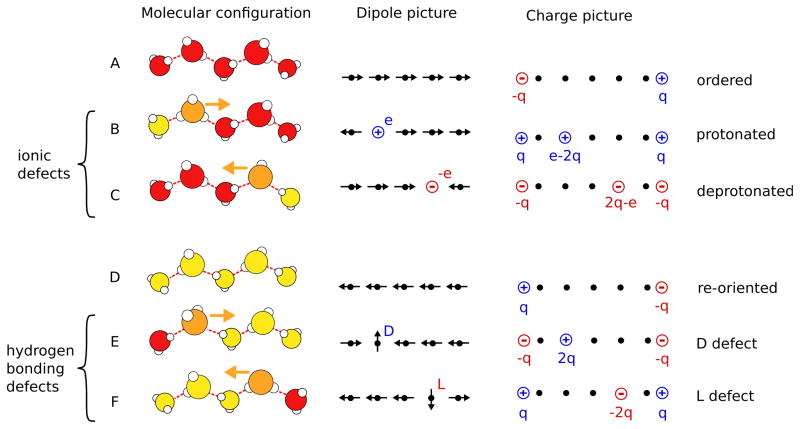
Fig. 4.
A Transport mechanisms of a protonic charge. Models with different levels of coarse graining are shown from the left to the right. Molecular configurations are shown in the left column, simplified dipole configurations in the middle column, and corresponding effective charges in the right column. Ordered chains are depicted in rows A and D, protonic defects in rows B and C, and hydrogen bonding defects in rows E and F. Water molecules are colored according to their dipole orientation with respect to the tube axis (red-right, yellow-left, orange-defect). To transport a positive charge from the left to the right the dipole moments of the water molecules in the chain have to point to the right (A). This chain can either transport a proton (depicted as a hydronium ion) from the left to the right (B) or a proton hole (hydroxide ion) from the right to the left (C) via structural diffusion, with the same net effect. In both cases, the translocation of the charge leads to the re-orientation of the chain (D). To complete the transport process and return to the original state of the chain, the chain has to be re-oriented in the original direction via the diffusion of either a D defect from the left to the right (E) or an L defect from the right to the left (F).