Abstract
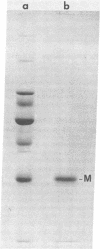
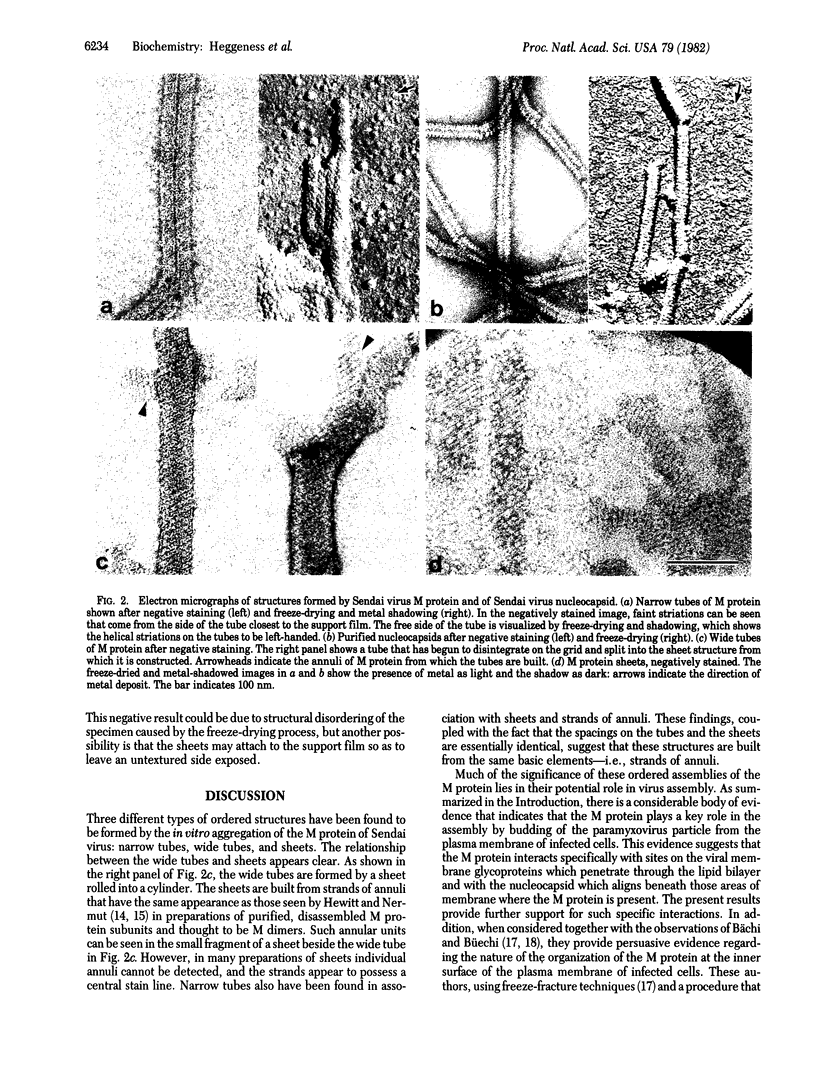
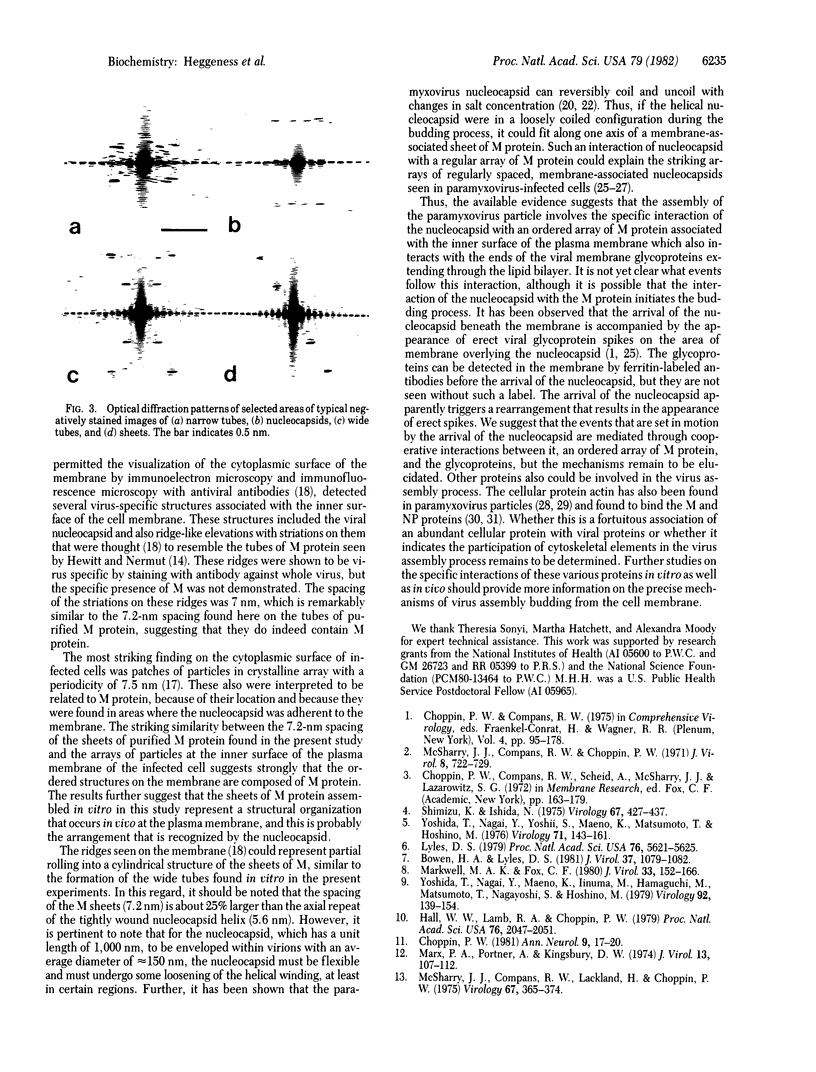
The nonglycosylated membrane protein (M) of Sendai virus was purified from virions and conditions were found under which the protein assembled in vitro into three types of ordered structures: narrow tubes, wide tubes, and sheets. These structures were examined by high resolution electron microscopy by using negative staining and metal shadowing techniques. The tubes and sheets are formed from strands 7.2 nm wide that are composed of annular subunits. The wide tubes appear to be formed by the rolling of a sheet into a cylinder in which the 7.2-nm strands are inclined with a pitch of 26-33 degrees and have a left-handed orientation. In addition to the strong reflections corresponding to the 7.2-nm spacings generated by the strands, optical diffraction patterns also showed weak reflections that could be indexed on a lattice corresponding to real-space lattice constants of 7.6 nm and 5.3 nm, with an included angle of 71 degrees. The dimensions and arrangements of these structures formed in vitro are strikingly similar to those of ordered arrays of particles found by others to be associated with the inner surface of the plasma membrane of infected cells. The results support the concept that ordered arrays of M protein, similar to those assembled in vitro, are involved in the assembly of the virus particle by budding from the cell membrane and that they provide specific recognition sites for the viral nucleocapsid at the cytoplasmic surface of the plasma membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- Bowen H. A., Lyles D. S. Structure of Sendai viral proteins in plasma membranes of virus-infected cells. J Virol. 1981 Mar;37(3):1079–1082. doi: 10.1128/jvi.37.3.1079-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T. Intramembrane structural differentiation in Sendai virus maturation. Virology. 1980 Oct 15;106(1):41–49. doi: 10.1016/0042-6822(80)90219-6. [DOI] [PubMed] [Google Scholar]
- Büechi M., Bächi T. Microscopy of internal structures of Sendai virus associated with the cytoplasmic surface of host membranes. Virology. 1982 Jul 30;120(2):349–359. doi: 10.1016/0042-6822(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Measles virus and chronic neurological diseases. Ann Neurol. 1981 Jan;9(1):17–20. doi: 10.1002/ana.410090104. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Scheid A., Choppin P. W. Conformation of the helical nucleocapsids of paramyxoviruses and vesicular stomatitis virus: reversible coiling and uncoiling induced by changes in salt concentration. Proc Natl Acad Sci U S A. 1980 May;77(5):2631–2635. doi: 10.1073/pnas.77.5.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Scheid A., Choppin P. W. the relationship of conformational changes in the Sendai virus nucleocapsid to proteolytic cleavage of the NP polypeptide. Virology. 1981 Oct 30;114(2):555–562. doi: 10.1016/0042-6822(81)90235-x. [DOI] [PubMed] [Google Scholar]
- Hewitt J. A., Nermut M. V. A morphological study of the M-protein of Sendai virus. J Gen Virol. 1977 Jan;34(1):127–136. doi: 10.1099/0022-1317-34-1-127. [DOI] [PubMed] [Google Scholar]
- Hewitt J. A. Studies on the subunit composition of the M-protein of Sendai virus. FEBS Lett. 1977 Sep 15;81(2):395–397. doi: 10.1016/0014-5793(77)80562-0. [DOI] [PubMed] [Google Scholar]
- Howe C., Morgan C., de Vaux St Cyr C., Hsu K. C., Rose H. M. Morphogenesis of type 2 parainfluenza virus examined by light and electron microscopy. J Virol. 1967 Feb;1(1):215–237. doi: 10.1128/jvi.1.1.215-237.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P. A., Portner A., Kingsbury D. W. Sendai virion transcriptase complex: polyeptide composition and inhibition by virion envelope proteins. J Virol. 1974 Jan;13(1):107–112. doi: 10.1128/jvi.13.1.107-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Lackland H., Choppin P. W. Isolation and characterization of the nonglycosylated membrane protein and a nucleocapsid complex from the paramyxovirus SV5. Virology. 1975 Oct;67(2):365–374. doi: 10.1016/0042-6822(75)90438-9. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba T., Hosaka Y., Sakiyama F. Isolation of the M polypeptide of Sendai virus (HVJ) with column chromatography. Biken J. 1977 Jun;20(2):77–80. [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Tyrrell D. L., Ehrnst A. Transmembrane communication in cells chronically infected with measles virus. J Cell Biol. 1979 May;81(2):396–402. doi: 10.1083/jcb.81.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y., Maeno K., Iinuma M., Hamaguchi M., Matsumoto T., Nagayoshi S., Hoshino M. Studies on the role of M protein in virus assembly using a ts mutant of HVJ (Sendai virus). Virology. 1979 Jan 15;92(1):139–154. doi: 10.1016/0042-6822(79)90220-4. [DOI] [PubMed] [Google Scholar]