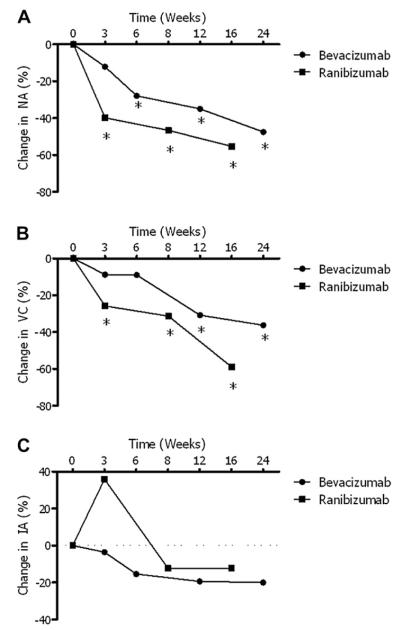
Figure 4.
Summary and comparison of the primary study metrics. (A) Neovascular area (NA): the ranibizumab treated cohort experienced a significant decrease by week 3, while the bevacizumab treated cohort required 6 weeks to experience a significant decrease; (B) vessel caliber (VC): ranibizumab treated patients experienced an earlier significant reduction (3 weeks) than bevacizumab treated patients (12 weeks); (C) invasion area (IA): neither medication produced a significant decrease at any time point. (*P ≤ 0.05 as compared to baseline measures.)