Abstract
Objective:Namibia faces a dual burden of HIV/AIDS and tuberculosis (TB). In 2010, HIV prevalence was 18.8%, the TB case notification rate was 634 cases per 100,000 population and the TB/HIV co-infection rate was 58%. There were 372 cases of drug-resistant TB (DR-TB) in 2009. The objective of this study was to assess the prevalence, profile and outcome of adverse events (AEs) associated with treatment of DR-TB and to explore possible influences of HIV disease on the occurrence of adverse events.
Methods:This was a cross-sectional descriptive study. After ethical approval, data were collected from treatment records of all patients treated for DR-TB at the study facility between January 2008 and February 2010 using a structured data collection form.
Results: A total of 141 adverse events of varying severity were experienced in 90% (53/59) of patients.The TB/HIV co-infection rate was 53% (n=31). The prevalence of gastrointestinal tract adverse events (abdominal pains, constipation, diarrhea, nausea and vomiting) was 64%, tinnitus 45%, joint pain 28%and decreased hearing 25%. Abdominal pains, rash, nausea, decreased hearing and joint pain were more common in HIV infected than in HIV uninfected patients.
Conclusions:Adverse events of varying severity are common during treatment of DR-TB, particularly in the intensive phase of therapy. Some adverse events were more prevalent in DR-TB patients co-infected with HIV. The study concludes that the characteristics and risk factors of serious adverse events should be further examined.
Keywords: tuberculosis, drug resistance, second-line drugs, adverse events, Namibia
Introduction
Tuberculosis (TB) exerts a huge burden of disease in Namibia, with a case notification rate (CNR) of 634 cases per 100,000 population in 2009 [1]. This is one of the highest tuberculosis CNRs in Africa. The TB/HIV co-infection rate was 58% in 2009 [1, 2]. Resistance to first-line regimens is a growing issue and could be due to various factors, including sub-optimal patient adherence to treatment schedules and defaulting in treatment [3]. Namibia reported 372 cases of drug resistant TB (DR-TB) in 2009, of which 74% of cases were`multi-drug resistant TB (MDR-TB), 22% poly-drug resistant TB and 5% were extensively drug resistant TB (XDR-TB) [1].
Although a number of studies [4-15] have examined the occurrence and characteristics of adverse events among patients on second-line anti-TB medicines, very few have specifically examined occurrence of adverse events in sub-Saharan Africa [16], especially in the context of high HIV prevalence and high TB/HIV co-infection rates. Most reviewed studies have mainly focused on adverse events of either one or two anti-TB medicines, but not on the entire treatment regimen [4-16].
This study describes the epidemiology of adverse events associated with treatment of DR-TB in a sub-Saharan country with a dual burden of TB and HIV. It further explores possible influences of HIV disease and antiretroviral treatment on the occurrence of adverse events.
The study thereby contributes to the existing body of epidemiologic and public health knowledge about treatment of DR-TB, focusing on a sub-Saharan country. This will assist managers of tuberculosis control programs, clinicians, and patients in similar socio-economic and epidemiologic settings in making evidence-based decisions for optimizing treatment outcomes for DR-TB patients, particularly in HIV co-infected patients. In this context, we aimed at assessing the profile, frequency and outcomes of adverse events associated with the use of second-line anti-TB medicines. The specific objectives of the study were:
To determine the types and frequency of adverse events associated with the use of second-line anti-TB medicines in a selected DR-TB treatment facility in Namibia.
To describe the characteristics, duration and outcomes of the adverse events, focusing on differences in adverse event occurrence between HIV infected and HIV uninfected persons.
Methods
Settings
The study was conducted in a 25-bed district hospital DR-TB ward with the second largest number of patients on DR-TB treatment in Namibia. Patients diagnosed with DR-TB are hospitalized in this TB ward, which is physically isolated from the rest of the wards in the hospital. This isolation is part of the infection control measures put in place at the facility to minimize nosocomial transmission of Mycobacteria tuberculosis. The patients with DR-TB infection are initiated on second-line treatment for about six months of intensive chemotherapy that includes injectable agents (amikacin, kanamycin or capreomycin). Until 2008, amikacin was the preferred aminoglycoside but this was later changed to kanamycin from 2009 onwards. The daily patient doses for each medicine used in the regimen were calculated and individualized according to the recommended World Health Organisation (WHO) body weight-based dosing scheme for anti-TB drugs (Table 3). Continuation therapy using oral anti-TB agents that includes a fluoroquinolone is maintained through an outpatient directly observed treatment short-course (DOTS)-plus programme. This DOTS-plus treatment is implemented through the health center closest to the patient. Patients on continuation therapy visit the health facility every day (Monday - Friday) for daily doses of second-line anti-tuberculosis medicines. Doctors and nurses elicit information on adverse events from patients and record them on a structured, pre-printed DR-TB treatment side effects monitoring form.
Study participants and data collection
For this cross-sectional descriptive study, the study population included all patients treated with second-line anti-TB medicines at the DR-TB treatment facility from 01 January 2008 to 24 February 2010. Treatment records were reviewed for all the patients treated for DR-TB during this period. Further, data on patient demographics, Mycobacterium tuberculosis drug resistance, medications and other clinical variables, including occurrence of adverse events and the characteristics of the adverse events, were collected from patient records using a structured data collection form. Since the present study did not involve direct contact with patients, informed patient consent was not required. Ethical approval of the study protocol was obtained from the research unit of the Ministry of Health and Social Services of Namibia (MoHSS) and the Higher Degrees Committee of the University of the Western Cape, South Africa.
Occurrence of adverse events and the analysis of data
The main outcome variable was the occurrence of adverse events. Further, a detailed characterization of the adverse events was conducted, which included: the adverse event description, time to onset of the adverse event, grading of severity of the adverse event, duration of the adverse event, actions taken to clinically manage the adverse event, and the outcome of the adverse event. Data were single-entered into Epi Info version 3.5.3 and the accuracy of entry verified against the original paper forms. The data were further checked for any errors and then analyzed using descriptive statistics. Absolute and relative frequency counts and measures of central tendency (mean, median and mode) were calculated. Measures of dispersion including range, interquartile range and standard deviation were also calculated. Student’s T-tests were used to assess differences in age and weight between the genders. A P-value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using Epi Info version 3.5.3., while Microsoft Excel® (2010) was used to draw charts.
Results
Fifty-nine (59) patients were treated for DR-TB during the study period. There were more male patients than females (66% vs. 34%). The mean patient age was 34.7 ± 9.4 (SD) years (Table 1). Males were slightly older than females (36.9 versus 31 years;P=0.02). The mean baseline weight was 52.5 ± 11.3 (SD) kilograms (kg), with no statistically significant gender difference (53.6 ± 7.8 kg males, versus 49.8 ± 16.4 kg females; P=0.23). About one-third of patients were unemployed.
Table 1. Demographic and clinical characteristics of the 59 patients treated with DR-TB therapy
| Characteristic | n (%) |
| Gender | |
| Male | 38 (64%) |
| Female | 20 (34%) |
| Missing | 1 (2%) |
| Age (years), SD | 34.7 ± 9.4 |
| Male | 36.9 ± 8.4 |
| Female | 31.0 ± 10.2 |
| Weight (kg), SD | 52.5 ± 11.3 |
| Male | 53.6 ± 7.8 |
| Female | 49.8 ± 16.4 |
| Occupation | |
| Unemployed | 18 (31%) |
| Employed | 20 (34%) |
| Student | 1 (2%) |
| Missing | 20 (34%) |
| Type of TB | |
| PTB smear + | 55 (93%) |
| PTB smear - | 3 (5%) |
| EPTB | 1 (2%) |
| Diagnostic category of DR-TB | |
| Previously treated with 1st line medicines | 46 (78%) |
| Previously treated with 2nd line medicines | 8 (14%) |
| New patient, never treated for TB | 5 (8%) |
| TB drug resistance pattern | |
| MDR | 36 (61%) |
| Poly resistant | 18 (28%) |
| XDR | 1 (2%) |
| Missing | 4 (6%) |
| Number of medicines in anti-TB regimen; median (range) | |
| Intensive phase regimens | 5 (4-7) |
| Continuation phase regimens | 3 (3-5) |
| Days on intensive phase treatment; Median (IQR) n=53 | |
| Male | 182 (154-186) |
| Female | 184 (165-211) |
| Days on continuation phase treatment; Median (IQR) n=49 | |
| Male | 389 (185-503) |
| Female | 522 (451-584) |
| HIV co-infection | 31 (53%) |
| Male | 19 (32%) |
| Female | 12 (20%) |
| Unknown | 3 (5%) |
| Proportion of HIV positive persons on HAART* | 13 (42%) |
| D4T/3TC/EFV | 5 (16%) |
| AZT/3TC/EFV | 3 (10%) |
| AZT/3TC/NVP | 2 (6%) |
| TDF/3TC/EFV | 2 (6%) |
| D4T/3TC/NVP | 1 (3%) |
* As percentage of number of patients with HIV co-infection
SD=standard deviation; kg=kilogrammes; TB=tuberculosis; PTB=pulmonary tuberculosis; + = positive; - = negative; EPTB=extra pulmonary tuberculosis; MDR=multidrug-resistant; XDR=extensively drug-resistant; IQR=interquartile range; HIV=human immunodeficiency virus; HAART= highly active antiretroviral therapy; d4T=stavudine; AZT=zidovudine; 3TC=lamivudine; EFV=efavirenz; TDF=tenofovir disoproxil fumarate; NVP=nevirapine
Almost all (92%) of the 59 patients had a prior history of treatment with either first-line or second-line anti-tuberculosis medicines. Approximately half of the patients (31/ 59 or 53%) were co-infected with the human immuno deficiency virus (HIV). Of the 31 HIV co-infected TB patients, 13 (42%) were on highly active antiretroviral treatment (HAART).
In total, there were fifteen different anti-tuberculosis medicines that were used by the patients included in this study (Table 3). Most of the patients were treated with DR-TB regimens containing pyrazinamide (93%) and ethionamide (92%). All patients were treated with an injectable anti-tuberculous agent (amikacin, kanamycin or capreomycin) during the intensive phase of treatment,with kanamycin being the most frequently used aminoglycoside in 54% of the patients. Fluoroquinolones (ciprofloxacin and levofloxacin) were used in almost all of the patients (98%), of which levofloxacin was used twice as much as ciprofloxacin (66% versus 32%).
There were 30 individualized regimens that were used in the intensive phase of treatment and 18 in the continuation phase of treatment. These individualized regimens were determined according to the drug sensitivity patterns of the infecting Mycobacterium tuberculosis strain.
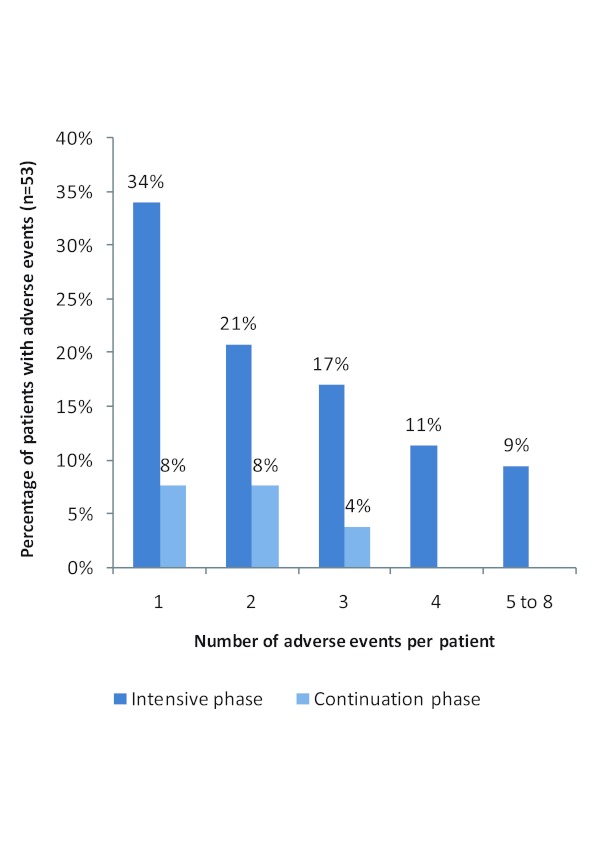
Fifty-three of the 59 patients experienced at least one adverse event of varying severity grading (90% prevalence). A total of 141 adverse events were reported by these patients. The number of adverse events experienced by an individual patient ranged from one to eight. The proportion of patients experiencing a given number of adverse events dramatically reduced from the intensive to the continuation phase of treatment (Figure 1).
Figure 1. Distribution of percentage of patients by number of adverse events experienced per patient in the intensive and continuation phases of treatment.
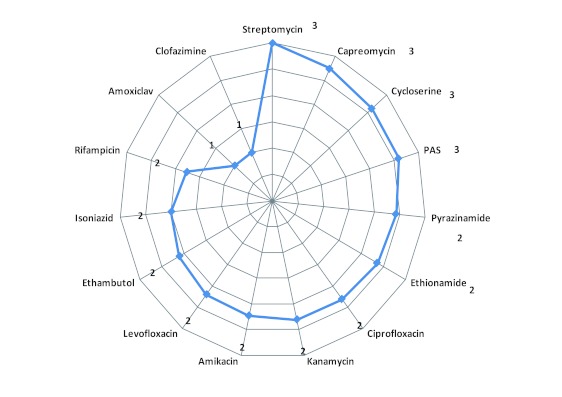
The average number of adverse events experienced by patients treated using specific anti-tuberculosis medicines ranged from one to three (Figure 2). Patients using regimens that contained streptomycin, capreomycin, cycloserine, and para-amino salicylic acid (PAS) experienced the highest average number (3) of adverse events, while patients using amoxycillin/ clavulanic acid and clofazimine experienced the fewest, with an average of one adverse event per drug. The rest of the medicines were associated with a similar average number of two adverse events per patient (Figure 2).
Figure 2. Average number of adverse events experienced per patient exposed to specific anti-tuberculosis drug.
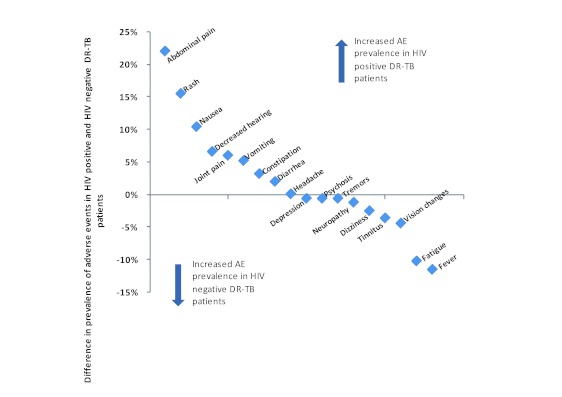
Hearing loss (decreased hearing), tinnitus, gastrointestinal tract (GIT)-related events (nausea, abdominal pains, vomiting, diarrhea and constipation) and joint pain were the predominant adverse events (Table 2). Five adverse events were more prevalent in HIV infected patients than in HIV uninfected patients (the figures in brackets show the excess frequency of occurrence in HIV infected patients as compared to HIV negative patients). These adverse events were: abdominal pains (22%); rash (16%); nausea (10%); decreased hearing (7%) and joint pain (6%). Contrarily, fever and fatigue are examples of adverse events that were reported less frequently by these patients (Figure 3).
Table2: Frequency of adverse events in both treatment phases; intensive and continuation phases respectively
| Grouped adverse events | Specific adverse events | Both phases (N=53)* |
% | Intensive phase (N=53) |
% | Continuation phase (N=49)† |
% |
| Hearing loss & Tinnitus | Tinnitus | 24 | 45% | 21 | 40% | 3 | 6% |
| Decreased hearing | 13 | 25% | 12 | 23% | 1 | 2% | |
| Hearing loss & Tinnitus Total | 37 | 70% | 33 | 62% | 4 | 8% | |
| GIT-related | Nausea | 12 | 23% | 8 | 15% | 4 | 8% |
| Abdominal pain | 9 | 17% | 8 | 15% | 1 | 2% | |
| Vomiting | 6 | 11% | 6 | 11% | 0 | 0% | |
| Diarrhea | 5 | 9% | 5 | 9% | 0 | 0% | |
| Constipation | 2 | 4% | 2 | 4% | 0 | 0% | |
| GIT Total | 34 | 64% | 29 | 55% | 5 | 10% | |
| Others | Joint pain | 15 | 28% | 13 | 25% | 2 | 4% |
| Headache | 11 | 21% | 10 | 19% | 1 | 2% | |
| Fatigue | 10 | 19% | 8 | 15% | 2 | 4% | |
| Dizziness | 8 | 15% | 7 | 13% | 1 | 2% | |
| Rash | 7 | 13% | 7 | 13% | 0 | 0% | |
| Neuropathy | 4 | 8% | 2 | 4% | 2 | 4% | |
| Fever | 3 | 6% | 3 | 6% | 0 | 0% | |
| Vision changes | 3 | 6% | 2 | 4% | 1 | 2% | |
| Depression | 2 | 4% | 2 | 4% | 0 | 0% | |
| Psychosis | 2 | 4% | 2 | 4% | 0 | 0% | |
| Severe hepatitis | 1 | 2% | 1 | 2% | 0 | 0% | |
| Decreased urine | 1 | 2% | 1 | 2% | 0 | 0% | |
| Anemia | 2 | 4% | 2 | 4% | 0 | 0% | |
| Loss of libido, delayed ejaculation | 1 | 2% | 0 | 0% | 1 | 2% | |
| Total of all adverse events | 141 | 122 | 19 | ||||
| Percent of all adverse events | 100% | 87% | 13% | ||||
* 53 of the 59 patients reported to have experienced at least one DR-TB treatment-related adverse event. All the 53 patients had either completed or were still in the intensive phase of treatment at the time of data collection.
† 49 of the patients had progressed into the continuation phase of treatment and were either still on continuation phase treatment or had completed treatment at the time of data collection.
%= percent. Sum of column percentages may exceed 100% because a patient may experience more than one adverse event. GIT = gastrointestinal tract
Table3: Prevalence of use and the weight-based dosing of specific anti-tuberculosis drugs in the treatment of drug-resistant tuberculosis in Namibia
| Drug name | DRUG EXPOSURE | DOSING BY WEIGHT CLASS | ||||
| Number of patients | Percent (n=59) | <33 KG | 33–50 KG | 51–70 KG | >70 KG (Maximum dose) | |
| Pyrazinamide | 55 | 93% | 30–40 mg/kg , daily | 1000–1750 mg, daily | 1750– 2000 mg , daily | 2000– 2500 mg ,daily |
| Ethionamide | 54 | 92% | 15–20 mg/kg daily | 500 mg | 750 mg | 750–1000 mg |
| Levofloxacin | 39 | 66% | Usual adult dose is 750 mg | 750 mg | 750 mg | 750–1000 mg |
| Ethambutol | 36 | 61% | 25 mg/kg , daily | 800–1200 mg, daily | 1200– 1600 mg , daily | 1600– 2000 mg daily |
| Kanamycin | 32 | 54% | 15–20 mg/kg daily | 500–750 mg | 1000 mg | 1000 mg |
| Cycloserine | 29 | 49% | 15–20 mg/kg daily | 500 mg | 750 mg | 750–1000 mg |
| Amikacin | 21 | 36% | 15–20 mg/kg daily | 500–750 mg | 1000 mg | 1000 mg |
| Ciprofloxacin | 19 | 32% | 20–30 mg/ kg daily | 1500 mg 1500 mg | 1500 mg | |
| Rifampicin | 13 | 22% | 10–20 mg/kg, daily | 450–600 mg, daily | 600 mg, daily | 600 mg, daily |
| Para-aminosalicylic acid | 5 | 8% | 150 mg/kg daily | |||
| Capreomycin | 4 | 7% | 15–20 mg/kg | 500–750 mg | 1000 mg | 1000 mg |
| Isoniazid | 4 | 7% | 4–mg/kg daily | 200–300 mg daily | 300 mg daily | 300 mg daily |
| or 8–12 mg, 3 x wk | or 450–600 mg, 3 x wk | or 600 mg , 3 x wk | or 600 mg, 3 x wk | |||
| Streptomycin | 3 | 5% | 15–20 mg/kg daily | 500–750 mg | 1000 mg | 1000 mg |
| Clofazimine | 1 | 2% | Efficacy and dosing in the treatment of drug-resistant TB not fully determined | |||
| Amoxicillin/Clavulanate | 1 | 2% | Efficacy and dosing in the treatment of drug-resistant TB not fully determined | |||
Source: WHO, (2006). Guidelines for the programmatic management of drug-resistant tuberculosis: 147-8.
mg = milligrammes; Kg = kilogrammes; wk = week
Figure 3. Comparison of difference in prevalence of adverse events in HIV positive and HIV negative DR-TB patients.
Fourteen (93%) of the 15 reported cases of joint pain were observed in patients treated with pyrazinamide-containing regimens.
Seventy three percent of the moderate-to-severe adverse events lasted for more than three (3) months, while 60% of the mild adverse events resolved within 3 months of onset. Overall, in 53% of patients, the adverse events resolved within 3 months of onset, while 47% of patients experienced adverse events that persisted beyond 3 months. Adverse events were severe and warranted discontinuation of the suspected offending medicine in four (4) out of 26 (15%) patients. Four (4) out of the 42 (9%) patients for whom data was available recovered from their adverse reactions with sequelae.
Discussion
Adverse events of varying severity, particularly tinnitus, hearing loss, GIT-related adverse events and joint pains were experienced by most (90%) of the patients included in this study. Most of the adverse events were reportedly experienced in the intensive phase of DR-TB treatment. Some differences in the occurrence of adverse events were observed between patients who were HIV infected and those who were HIV uninfected. Abdominal pains, rash, nausea, decreased hearing and joint pain were among the adverse events more frequently reported by HIV infected patients, whereas fever and fatigue were reported relatively less frequently, when compared with HIV uninfected patients.
The 90% prevalence of adverse events observed in the current study is higher than that reported in other studies, where it ranged from 69%-86% [4-14, 16]. It was slightly lower than the 96% reported by Tupasi and colleagues in their study of 117 patients in the Philippines [15]. The reasons for the heterogeneity in the prevalence of adverse events across the various studies is unclear, but might be related to several possible factors such as: differences in definitions of adverse events terminologies across settings, whether the adverse event was symptomatic and patient-reported (subjective) or clinician-validated (objective), whether all or only the severe and serious adverse events were studied, variations in the use of specific anti-TB agents, and/or the differences in co-morbidities and other covariates between study settings. Our study’s cohort is similar to other cohorts in terms of demographics and number of anti-TB medicines used and treatment duration. In addition, treatment was according to existing guidelines [3, 17]. However, the HIV co-infection rate and the specific anti-TB agents used may differ between settings and this should be borne in mind when interpreting and comparing results of adverse events reported from different countries. Although the present study found the TB/HIV co-infection rate to be higher than that reported in Europe and South East Asia (where HIV prevalence rates are low) [6,13,18], it is lower than that observed for Lesotho, a country in Southern Africa, which has a high prevalence of HIV infection [16].
The frequency of tinnitus (45%) in the present study was higher than the 5.1% - 24% range reported in the literature [4, 14, 15], while that of hearing loss (25%) was within the range of 6.7% - 33% reported in the literature [5, 11, 14, 15]. From the review of the literature, the reported rates of ototoxicity (tinnitus and hearing loss) ranged from 12% to 42% [6, 7, 16]. Our study found an almost double rate of ototoxicity, when compared to the 36% reported by Seung et al. [16], whose study population and HIV prevalence rates are similar to our population. It is unclear why this is so, but one possible reason could be that the majority of patients in the Seung study were still in the early stages of treatment, hence not all potential adverse events may have occurred by the time of completion of their study. The high degree of heterogeneity of ototoxicity observed in the literature could have been brought about by differences in the use of specific ototoxic anti-TB agents, as well as by the differences in the profiles of co-morbidities in the different patient population groups of the various studies.
Ototoxicity (tinnitus and decreased hearing) is predominantly associated with the use of parenteral anti-tuberculous agents (aminoglycosides and aminopeptides) [19-24]. The drug-specific rate of patient-reported tinnitus in the current study ranged from 33%- 50%, while hearing loss was 13% - 67%. These findings are above the range of 15.4% - 33% reported in studies conducted elsewhere [5, 19, 20]. The high prevalence of tinnitus and hearing loss found in our study is probably because they were symptomatic or patient-reported (subjective) and may not have been clinically validated by audiometric tests. In addition, there could have been additive effects of interaction with other concomitant and potentially ototoxic anti-TB drugs that were used in the anti-TB regimens, such as fluoroquinolones and cycloserine. Additionally, there are possibilities of interactive effects from HIV disease and the concomitant use of antiretroviral medicines, which may have contributed to this high rate of ototoxicity. This needs further investigation to uncover the possibility of these interactive effects.
The gastrointestinal tract (GIT)-related adverse events were the second most observed group of adverse events, reported by 64% of the patients. The specific GIT-related adverse events were: nausea (23%), abdominal pain (17%), vomiting (11%), diarrhea (9%), and constipation (4%). The frequency of occurrence of these specific GIT-related adverse events fall within the wide range (10.8% - 100%) which has been reported in the literature [4, 6, 7, 11, 14, 15, 16]. Since some studies have reported higher rates of specific GIT-related adverse events, it is possible that patients in our study may have selectively under-reported these adverse events during the course of their treatment.
The possibility of drug-drug interactions [10], drug-disease and disease-disease interactions should be reflected on in the present study, particularly considering that an average of five different anti-TB agents were used by each patient in the study and that over 50% of the patients had HIV co-infection, 42% of whom were on concomitant antiretroviral medication.
In our study, adverse events were severe and warranted discontinuation of the suspected offending medicine in 15% of patients. This prevalence of treatment discontinuation is lower than that reported in the literature [4, 5, 12, 14]. Generally, our findings are similar to the findings of Furin et al. (2001) that adverse events of the anti-TB medicines were bearable and did not cause discontinuation of the treatment apart from the occasional suspension of an offending agent in 11.7% of the patients [11].
Strength of the study
The data used in this study reflect real-life DR-TB treatment practices and patient experiences. The cross-sectional descriptive design enabled us to examine and describe the prevalence and profile of adverse events in the patient sample. We were able to generate a tentative hypothesis that some adverse events occur more in DR-TB patients co-infected with HIV, which is clinically important when treating this sub-group of patients.
Limitation of the study
By using retrospective data, we encountered instances of missing patient treatment records and missing data on specific variables. Furthermore, it was not possible to perform qualitative causality assessment of the adverse events using the available data, especially given the paucity of laboratory data. The adverse events recorded on the patients’ side-effects monitoring form were based on patient-reported symptoms. Hence, there was a possibility of subjectivity and of selective under-reporting of adverse events by patients or the selective recording of adverse events by clinicians, which may have biased the results away from the true prevalence. Some symptoms of reported adverse events may have overlapped with symptoms of HIV/ AIDS. The small sample size and the use of data from one facility may not allow for generalization of findings beyond the studied sample.
Conclusion
This study found that adverse events, of varying severity, most commonly occur in the intensive phase of DR-TB treatment.While most patients tolerated the second-line anti-TB medicines used in Namibia’s DR-TB treatment program, about 10% of patients experienced serious adverse events, with a possibility of suffering permanent disability. Some adverse events were more prevalent in DR-TB patients co-infected with HIV. The characteristics, magnitude of risk and risk factors of these serious and potentially permanent adverse events should be thoroughly examined and elucidated in subsequent prospective active surveillance pharmacovigilance or cohort studies. Therefore, clinicians, including pharmacists, should closely monitor and aggressively manage adverse events during the intensive phase of DR-TB treatment and should always consider the possibility of increased occurrence of adverse events in patients co-infected with HIV.
Authors’ contributions
Evans Sagwa conceived and designed the study; collected, analyzed the data, drafted and finalized the manuscript. Brian van Wyk, Panganai Dhliwayo, Nunurai Ruswa, and Jean Paul Musasa reviewed the study protocol and manuscript. Aukje Kaija Mantel-Teeuwisse and Shanthi Pal critically reviewed the manuscript.
Conflict of interest
None
Acknowledgments
The authors would like to thank H.G.M Leufkens, J. Rohde, F. Mavhunga, M.Malakia, E. Moreno, A. Mengistu, C. Corbell, J. Nwokike, D. Mabirizi, A. Stergachis , R. Laing and T. Rennie for their contributions in this study. Special thanks to all the faculty members of the Pan African Thoracic Society course in methods for epidemiologic, clinical and operational research (PATS-MECOR) for their technical assistance in the interpretation of the study findings. Tuberculosis patient care and treatment is a Government and donor funded service freely provided by health facilities of the Ministry of Health and Social Services, Namibia.
Funding Statement
Funding of this study was provided by Evans Sagwa as part of his research towards the Master of Public Health degree, University of the Western Cape, South Africa.
References
- 1.Health and Social Services (MoHSS). National Tuberculosis and Leprosy Programme: Second Medium Term Strategic Plan for Tuberculosis and Leprosy. National Tuberculosis and Leprosy Programme: Second Medium Term Strategic Plan for Tuberculosis and Leprosy, 2010-2015. Windhoek: Ministry of Health and Social Services (MoHSS); 2010. [Google Scholar]
- 2.Report on the National HIV Sentinel Survey. Windhoek: Ministry of Health and Social Services; 2008. [Google Scholar]
- 3.National Guidelines for the Management of Tuberculosis. Windhoek: Ministry of Health and Social Services; 2006. [Google Scholar]
- 4.Nathanson E, Gupta R, Huamani P, Leimane V, Pasechnikov A D, Tupasi T E, Vink K, Jaramillo E, Espinal M A. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2005;8(11):1382–4. [PubMed] [Google Scholar]
- 5.Tahaoğlu K, Törün T, Sevim T, Ataç G, Kir A, Karasulu L, Ozmen I, Kapakli N. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med. 2001;345(3):170–4. doi: 10.1056/NEJM200107193450303. http://dx.doi.org/10.1056/NEJM200107193450303. [DOI] [PubMed] [Google Scholar]
- 6.Leimane Vaira, Riekstina Vija, Holtz Timothy H, Zarovska Evija, Skripconoka Vija, Thorpe Lorna E, Laserson Kayla F, Wells Charles D. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365(9456):318–26. doi: 10.1016/S0140-6736(05)17786-1. http://www.scholaruniverse.com/ncbi-linkout?id=15664227. [DOI] [PubMed] [Google Scholar]
- 7.Törün T, Güngör G, Ozmen I, Bölükbaşi Y, Maden E, Biçakçi B, Ataç G, Sevim T, Tahaoğlu K. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9(12):1373–7. http://www.nlm.nih.gov/medlineplus/antibiotics.html. [PubMed] [Google Scholar]
- 8.Cox HS, Kalon S, Allamuratova S, Sizaire V, Tigay ZN, Sabine R, Hamraev AK, Kebede Y, Mills C. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: Treatment complexity and XDR-TB among treatment failures. 2:e1126. doi: 10.1371/journal.pone.0001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahar BL, Mosharrof Hossain AKM, Islam MM, Saha DR. A comparative study on the adverse effects of two anti-tuberculosis drugs regimen in initial two-month treatment period. Bangladesh J Pharmacol. 2006;1:51–7. [Google Scholar]
- 10.Papastavros Tina, Dolovich Lisa R, Holbrook Anne, Whitehead Lori, Loeb Mark. Adverse events associated with pyrazinamide and levofloxacin in the treatment of latent multidrug-resistant tuberculosis. CMAJ. 2002;167(2):131–6. http://pubmedcentralcanada.ca/pmcc/articles/pmid/12160118. [PMC free article] [PubMed] [Google Scholar]
- 11.Furin J J, Mitnick C D, Shin S S, Bayona J, Becerra M C, Singler J M, Alcantara F, Castañieda C, Sanchez E, Acha J, Farmer P E, Kim J Y. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:648–55. [PubMed] [Google Scholar]
- 12.Shin S S, Pasechnikov A D, Gelmanova I Y, Peremitin G G, Strelis A K, Mishustin S, Barnashov A, Karpeichik Y, Andreev Y G, Golubchikova V T, Tonkel T P, Yanova G V, Yedilbayev A, Rich M L, Mukherjee J S, Furin J J, Atwood S, Farmer P E, Keshavjee S. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11(12):1314–20. http://www.scholaruniverse.com/ncbi-linkout?id=18034952. [PubMed] [Google Scholar]
- 13.Lanternier F, Dalban C, Perez L, Bricaire F, Costagliola D, Caumes E. Tolerability of anti-tuberculosis treatment and HIV serostatus. Int J Tuberc Lung Dis. 2007;11(11):1203–9. http://www.nlm.nih.gov/medlineplus/tuberculosis.html. [PubMed] [Google Scholar]
- 14.Bloss E, Kuksa L, Holtz T H, Riekstina V, Skripconoka V, Kammerer S, Leimane V. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000-2004. Int J Tuberc Lung Dis. 2010;14(3):275–81. [PubMed] [Google Scholar]
- 15.Tupasi Thelma E, Gupta Rajesh, Quelapio Ma Imelda D, Orillaza Ruth B, Mira Nona Rachel, Mangubat Nellie V, Belen Virgil, Arnisto Nida, Macalintal Lualhati, Arabit Michael, Lagahid Jaime Y, Espinal Marcos, Floyd Katherine. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the Philippines. PLoS Med. 2006;3(9):e352. doi: 10.1371/journal.pmed.0030352. http://pubmedcentralcanada.ca/pmcc/articles/pmid/16968123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seung Kwonjune J, Omatayo David B, Keshavjee Salmaan, Furin Jennifer J, Farmer Paul E, Satti Hind. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS One. 2009;4(9):e7186–10. doi: 10.1371/journal.pone.0007186. http://pubmedcentralcanada.ca/pmcc/articles/pmid/19779624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treatment of tuberculosis, WHO/HTM/TB/2009.420. Geneva: World Health Organization; 2010. [Google Scholar]
- 18.Cain K P, Kanara N, Laserson K F, Vannarith C, Sameourn K, Samnang K, Qualls M L, Wells C D, Varma J K. The epidemiology of HIV-associated tuberculosis in rural Cambodia. Int J Tuberc Lung Dis. 2007;11(9):1008–13. http://hivinsite.ucsf.edu/InSite?page=kb-05-01-01-01. [PubMed] [Google Scholar]
- 19.de Jager P, van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis. 2002;6(7):622–7. http://www.nlm.nih.gov/medlineplus/antibiotics.html. [PubMed] [Google Scholar]
- 20.Duggal Prahlad, Sarkar Malay. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. 5. 2007;7:5–1186. doi: 10.1186/1472-6815-7-5. http://www.biomedcentral.com/1472-6815/7/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brummett R E, Fox K E. Aminoglycoside-induced hearing loss in humans. Antimicrob Agents Chemother. 1989;33(6):797–800. doi: 10.1128/aac.33.6.797. http://pubmedcentralcanada.ca/pmcc/articles/pmid/2669624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadol J B. Medical progress: Hearing loss. N Engl J Med. 1993;329:1092–102. doi: 10.1056/NEJM199310073291507. [DOI] [PubMed] [Google Scholar]
- 23.Tan Kelvin H-V, Mulheran Michael, Knox Alan J, Smyth Alan R. Aminoglycoside prescribing and surveillance in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):819–23. doi: 10.1164/rccm.200109-012CC. http://ghr.nlm.nih.gov/condition=cystic-fibrosis. [DOI] [PubMed] [Google Scholar]
- 24.Selimoglu Erol. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13(1):119–26. doi: 10.2174/138161207779313731. http://www.nlm.nih.gov/medlineplus/antibiotics.html. [DOI] [PubMed] [Google Scholar]