Abstract
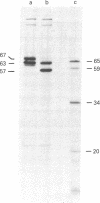
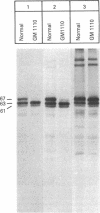
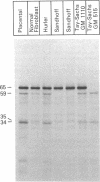
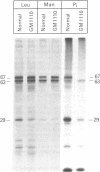
RNA was isolated from human term placenta or cultured fibroblasts and translated in a rabbit reticulocyte system in the presence of [35S]methionine; the translation products were immunoprecipitated with antisera made against beta-hexosaminidase or its isolated alpha and beta chains and analyzed by polyacrylamide gel electrophoresis. The largest translated alpha and beta chain polypeptides had Mrs of 65,000 and 59,000, respectively. These are approximately equal to 2,000 greater than the Mrs of precursor chains synthesized by intact fibroblasts and deglycosylated with endo-beta-N-acetylglucosaminidase H suggesting the presence of a signal sequence. RNA of fibroblast cultures from two patients with Sandhoff disease did not direct the translation of immunoprecipitable beta chain; RNA of fibroblast cultures from four patients with Tay-Sachs disease (three of Ashkenazi Jewish descent and one of non-Jewish descent) did not direct the translation of immunoprecipitable alpha chain. In contrast, a normal amount of alpha chain was made in the presence of RNA from the fibroblast culture of another non-Jewish Tay-Sachs patient (GM 1110). Intact fibroblasts from this patient also synthesized the alpha chain as shown by labeling with [3H]leucine; however, strong detergent was required for extraction. The alpha chain could be labeled with [3H]mannose but not with [32P]phosphate; it was neither secreted nor accumulated in the proteolytically processed form, and it disappeared within a day of synthesis. A plausible though not unique explanation is that the insoluble alpha chain is not transported from the endoplasmic reticulum (the site of glycosylation) to the Golgi apparatus (the site of phosphorylation) nor to further points of destination--lysosomes and the exterior of the cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boime I., McWilliams D., Szczesna E., Camel M. Synthesis of human placental lactogen messenger RNA as a function of gestation. J Biol Chem. 1976 Feb 10;251(3):820–825. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Erickson A. H., Conner G. E., Blobel G. Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem. 1981 Nov 10;256(21):11224–11231. [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Frisch A., Neufeld E. F. Limited proteolysis of the beta-hexosaminidase precursor in a cell-free system. J Biol Chem. 1981 Aug 10;256(15):8242–8246. [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hercz A., Katona E., Cutz E., Wilson J. R., Barton M. alpha1-Antitrypsin: the presence of excess mannose in the Z variant isolated from liver. Science. 1978 Sep 29;201(4362):1229–1232. doi: 10.1126/science.308696. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Larsson C., Eriksson S. Characterization of alpha1-antitrypsin in the inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med. 1975 Sep 18;293(12):576–579. doi: 10.1056/NEJM197509182931203. [DOI] [PubMed] [Google Scholar]
- Kaback M. M., Nathan T. J., Greenwald S. Tay-Sachs disease: heterozygote screening and prenatal diagnosis--U.S. experience and world perspective. Prog Clin Biol Res. 1977;18:13–36. [PubMed] [Google Scholar]
- Krangel M. S., Pious D., Strominger J. L. Human histocompatibility antigen mutants immunoselected in vitro. Biochemical analysis of a mutant which synthesizes an altered HLA-A2 heavy chain. J Biol Chem. 1982 May 10;257(9):5296–5305. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Braell W. A., Schwartz A. L., Strous G. J., Zilberstein A. Synthesis and assembly of membrane and organelle proteins. Int Rev Cytol Suppl. 1981;12:247–307. doi: 10.1016/b978-0-12-364373-5.50016-0. [DOI] [PubMed] [Google Scholar]
- Myrianthopoulos N. C., Aronson S. M. Population dynamics of Tay-Sachs disease. I. Reproductive fitness and selection. Am J Hum Genet. 1966 Jul;18(4):313–327. [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Waheed A., Hasilik A., von Figura K. Synthesis of phosphorylated recognition marker in lysosomal enzymes is located in the cis part of Golgi apparatus. J Biol Chem. 1982 May 25;257(10):5323–5325. [PubMed] [Google Scholar]
- Reitman M. L., Kornfeld S. UDP-N-acetylglucosamine:glycoprotein N-acetylglucosamine-1-phosphotransferase. Proposed enzyme for the phosphorylation of the high mannose oligosaccharide units of lysosomal enzymes. J Biol Chem. 1981 May 10;256(9):4275–4281. [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R. The mannose 6-phosphate receptor of Chinese hamster ovary cells. Compartmentalization of acid hydrolases in mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10623–10627. [PubMed] [Google Scholar]
- Rome L. H., Weissmann B., Neufeld E. F. Direct demonstration of binding of a lysosomal enzyme, alpha-L-iduronidase, to receptors on cultured fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2331–2334. doi: 10.1073/pnas.76.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Kreibich G., Popov D., Kato K., Sabatini D. D. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982 Apr;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Distler J., Jourdian G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4289–4293. doi: 10.1073/pnas.78.7.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Berg R. A., Rennard S. I., Bradley K. H., Trapnell B. C., Crystal R. G. Procollagen production and procollagen messenger RNA levels and activity in human lung fibroblasts during periods of rapid and stationary growth. J Biol Chem. 1981 Mar 25;256(6):3135–3140. [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Identification of a rat liver alpha-N-acetylglucosaminyl phosphodiesterase capable of removing "blocking" alpha-N-acetylglucosamine residues from phosphorylated high mannose oligosaccharides of lysosomal enzymes. J Biol Chem. 1980 Sep 25;255(18):8398–8401. [PubMed] [Google Scholar]
- Waheed A., Pohlmann R., Hasilik A., von Figura K. Subcellular location of two enzymes involved in the synthesis of phosphorylated recognition markers in lysosomal enzymes. J Biol Chem. 1981 May 10;256(9):4150–4152. [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]