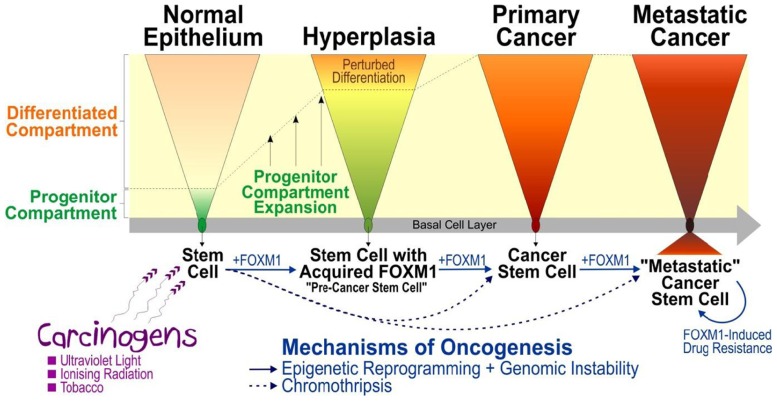
FIGURE 1.
A model mechanism illustrating the role of FOXM1 in human epithelial cancer initiation, progression, and metastasis. Multiple lines of evidence have suggested that carcinogens (such as ultraviolet light, ionizing radiation, tobacco, etc) exposure causes activation of FOXM1 which triggers aberrant expansion of “pre-cancer stem cells” through perturbation of epithelial differentiation, producing a premalignant hyperplastic phenotype (Gemenetzidis et al., 2010). Activation of genomic instability (e.g., through activation of CEP55 leading to mitotic instability; Gemenetzidis et al., 2009) and epigenetic reprograming (e.g., through deregulation of HELLS causing chromatin remodeling and altered genomic methylation) triggered by aberrant expression of FOXM1 (Teh et al., 2012) may predispose cells to further mutations thereby driving oncogenic progression and subsequent metastatic invasion. Due to the complexity of genomic instability and epigenetic reprograming activated by FOXM1, this model may explain the heterogeneity found in tumor whereby the initial molecularly distinct “pre-cancer stem cells” undergo constant adaptive evolutionary changes to produce “cancer stem cells” and subsequently “metastatic cancer stem cells” during the course of cancer progression. An alternative mechanism involving catastrophic genomic rearrangement, chromothripsis, has been shown to produce tumor directly and rapidly from normal cells without the need for sequential accumulation of oncogenic mutations (Liu et al., 2011; Stephens et al., 2011; Crasta et al., 2012).