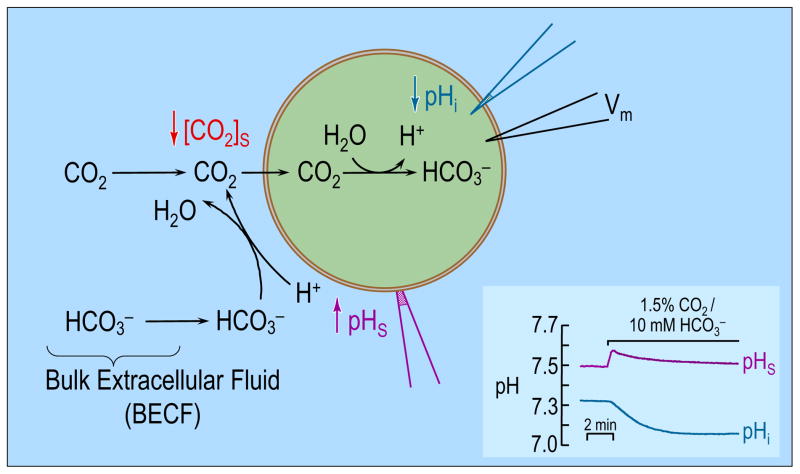
Figure 1.
Model of an oocyte exposed to . As CO2 enters the cell, the concentration of CO2 near the outer surface of the oocyte falls. The lost CO2 can be replenished by 1) diffusion from the bulk extracellular fluid (BECF) and 2) the reaction(s) at the cell surface. This reaction, which consumes H+, causes pHS to rise. Conversely, the entry of CO2 into the cell leads to the generation of intracellular H+ via the reaction(s) , therefore causing a fall in pHi. (Cell model adapted from ref (Boron, 2010). The inset at the bottom right shows experimental pHS and pHi records for a H2O-injected oocyte exposed to 1.5% CO2/10mM . Data kindly provided by Dr. Musa-Aziz).