Figure 1.
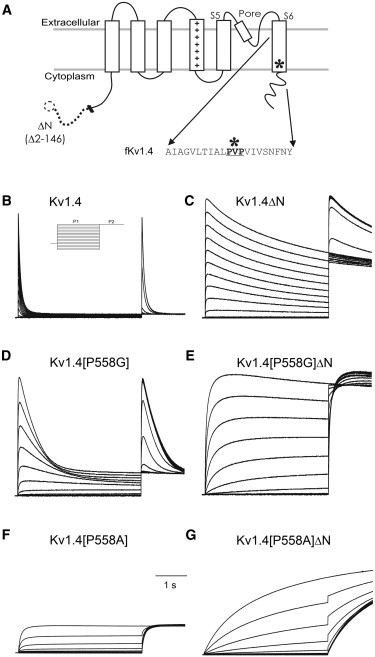
Mutations in Kv1.4. (A) Topological cartoon. Kv1.4 is thought be a tetramer of four subunits, each of which has six transmembrane segments, with a voltage sensor in S4. The position of the proline hinge (∗) and the sequence of the amino acids around it are shown. In the ΔN construct, amino acids 2–146 are removed from N-terminal lipophilic ball responsible for N-type inactivation. Representative traces from a two-pulse protocol on channels expressed in Xenopus oocytes. P1 (4 s) was to potentials from −100 and +50 mV, P2 (1 s) was to +50 mV. Holding potential: −90 mV. (B) Kv1.4 (inset: voltage protocol). (C) Kv1.4ΔN. (D) Kv1.4[P558G]. (E) Kv1.4[P558G]ΔN. (F) Kv1.4[P558A]. (G) Kv1.4[P558A]ΔN.
