Figure 1.
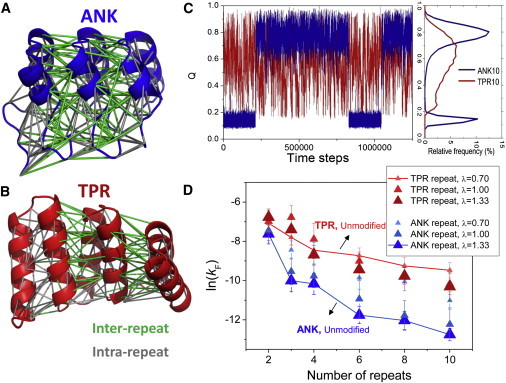
Structure and folding of ANK and TPR proteins. (A and B) The structures of three units of ANK (A, in blue) and TPR (B, in red). In both structures, each inter-and intrarepeat contact is shown in green and gray, respectively. The boundaries of each repeat unit are identical to those used in previous studies (30,31). (C) Folding reactions of ANK10 (blue) and TPR10 (red). Left: The fraction of the total number of native contacts (Q) that are formed during the folding reaction. Right: The relative frequency of Q, illustrating that ANK populates mostly two states, whereas various states of different Qs are significantly populated for TPR. (D) The folding rates (ln(kF); more-negative values indicate a slower rate) of a series of repeat proteins with modulated strength of interfacial contacts, which is tuned by λ (the ratio between the total strength of the interfacial and internal repeat contacts in the ANK and TPR series) are shown in blue and red, respectively. The value of λ is reflected by the relative size of the triangle and the strength of its color. The number of units of each member in the series (2–10 units) appears on the X axis. The connecting lines serve as a guide to the eye.
