Figure 4.
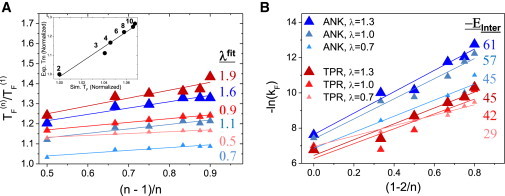
Fitting the simulated stabilities and folding rates of the modified ANK and TPR proteins to a simple capillarity model. (A) Correlation among folding stability, the number of repeat units (n), and the ratio between EInter and EIntra, λ. The folding temperatures (normalized by the TF of a protein with a single repeat) of each modified and unmodified TPR and ANK repeat (λ = 0.7, 1.0, and 1.3 in various systems) with a different number of repeat units (n) are plotted as a function of (n − 1)/n. All of the systems fit a linear function with correlation coefficients > 0.95. The slope of the linear fits, which corresponds to λ, is in very good agreement with the value of λ used in the simulation (c = 5). The inset shows a comparison between the experimental and simulated folding temperatures of a series of unmodified TPR proteins comprising 2–10 repeat units (correlation coefficient R = 0.96). (B) Correlation among the simulated folding rates, n, and the strength of the interface. The folding rate is plotted as a function of (1 − 2/n). The simulated data of the six series fit linear functions with correlation coefficients > 0.9. The slope of the linear fit, which corresponds to EInter, is similar to the value used in the simulations (when a is assigned a value of 10). The values of EInter in the simulation model are 58, 45, and 33 for ANK, and 58, 43, and 33 for TPR (where the large numbers refer to the systems with larger λ values). The color scheme is the same as that used in Fig. 1D.
