Figure 1.
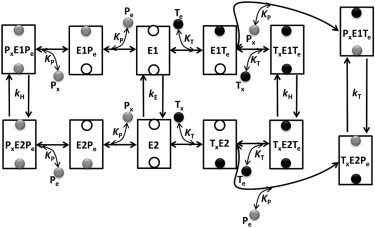
Proposed kinetic mechanism of ATP (MgATP2−) and Pi (HPO42−) exchange via the ATP-Mg/Pi carrier (APC). The mechanism is ordered bi-bi for heteroexchange and random bi-bi for homoexchange of ATP and Pi. It is assumed that the APC has two binding sites: one exposed to the external side and the other to the matrix side. Once both the binding sites are occupied, the APC undergoes conformational change to translocate the bound substrates. E1 is the unbound state of the APC to which either of the external substrates can bind first for homoexchanges, but only external ATP can bind first for heteroexchange. E2 state is analogous to E1 with the exception that matrix substrate can bind first. KT and KP represent the binding affinity of the APC for ATP and Pi, respectively; kE is the rate of futile exchange between the unbound states E1 and E2, kT is the translocation rate when ATP is exchanged for Pi, and kH is the translocation rate for homoexchanges. Here, Tx and Px represent matrix ATP and Pi, whereas Te and Pe represent external ATP and Pi.
