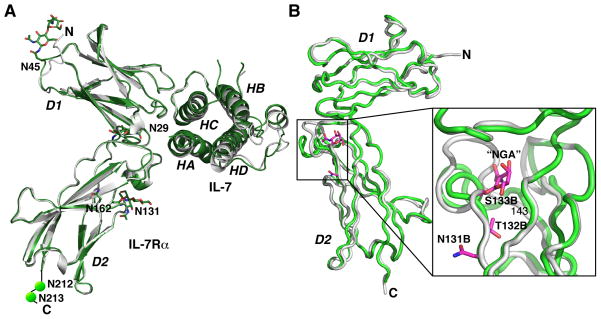
Fig. 5. Structural views of glycosylation of the IL-7Rα.
(A) Structural superimposition of the IL-7 complex with an unglycosylated IL-7Rα onto the IL-7 complex with a glycosylated IL-7Rα, indicating that N-linked glycosylation does not participate directly in the site 1 interface with IL-7 and does not induce large conformational changes between the two structures. The six asparagines of the IL-7Rα that can be N-linked glycosylated (N29, N45, N131, N162, N212, and N213) are labeled and two N-acetyl glucosamines attached to N29, N45, and N131 were experimentally observed. The secondary elements of the IL-7Rα molecules superimpose with an rmsd of 0.6 Å. (B) Experimental observation of an O-linked glycosylation of an N-acetyl galactosamine to S133 and the conformational changes induced by the glycan from residues 133-143 of the two IL-7Rα structures (15).