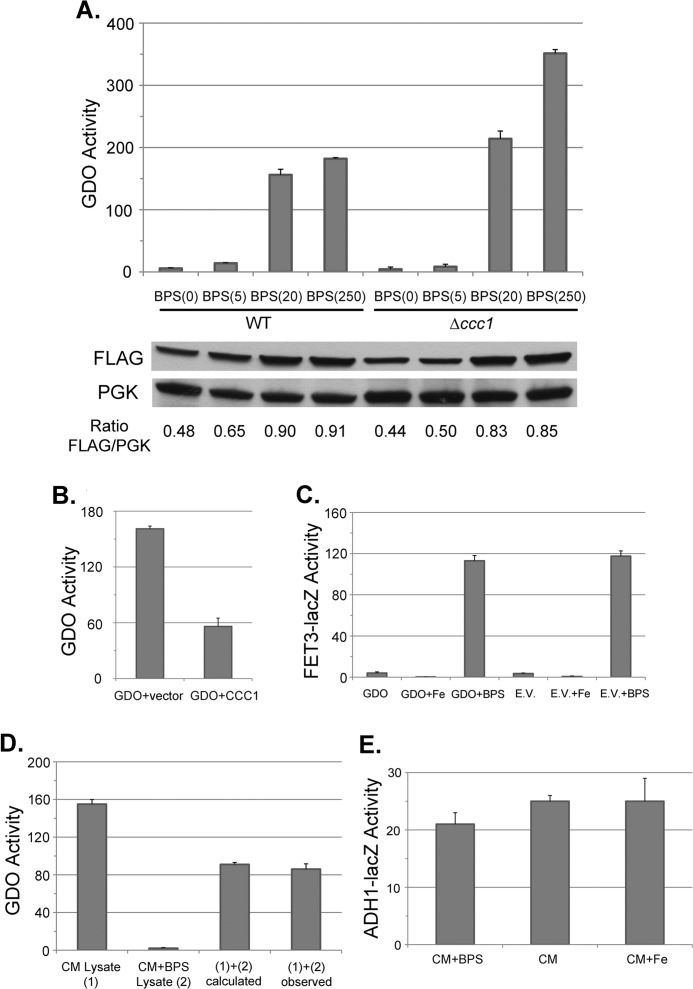
FIGURE 3.
The activity of expressed GDO in yeast can be used as a measure of cytosolic iron. A, wild type or Δccc1 cells were transformed with a plasmid expressing GDO with a carboxyl-FLAG epitope, under the control of the ADH1 promoter. Cells were grown in CM medium supplemented with 80 μm BPS and various concentrations of FeSO4 (i.e. BPS(5) indicates 80 μm BPS with 5 μm FeSO4) added back. Cells were grown overnight to mid-log phase and homogenized as described under ”Experimental Procedures,“ and GDO activity and cell protein were determined. GDO activity is expressed as nanomoles of substrate converted per minute per mg of protein. The amount of GDO-FLAG was detected by Western blot with phosphoglucose kinase (PGK) as a loading control. The ratio of GDO-FLAG to PGK was determined by densitometry. B, wild type cells were transformed with either a control plasmid or a plasmid containing CCC1 under the control of its endogenous promoter and a plasmid containing GDO under the control of the ADH1 promoter. Cells were grown overnight in selective medium and homogenized, and GDO activity and cell protein were determined. The data are expressed as specific activity, and the results are the mean of three separate experiments (± S.D.). C, wild type cells containing a FET3-lacZ reporter construct integrated at the HO locus were transformed with a GDO expression plasmid or an empty vector (E.V.). Cells were grown in CM medium with or without 40 μm BPS (+BPS) with 100 μm FeSO4 added (+Fe); cell lysates were prepared, and β-galactosidase activity and cell protein were determined. The data are presented as specific activity, and the results are the mean of three separate experiments (± S.D.). D, cells expressing GDO were grown overnight in CM medium without BPS added (CM Lysate (1)) or CM medium with 80 μm BPS added (CM+BPS Lysate (2)). Cells were harvested, and the lysates from these two conditions were assayed separately or were mixed together at a 1:1 volume ratio prior to determining GDO activity. The calculated value ((1)+(2) calculated) indicates the theoretical GDO activity assuming no activity change occurring upon mixing. The data are presented as specific activity, and the results are the mean of three separate experiments (± S.D.). E, WT cells (DY150) were transformed with a plasmid containing a ADH1-lacZ construct and grown in low iron medium (CM supplemented with 80 μm BPS, CM+BPS), regular medium (CM), or high iron (CM supplemented with 100 μm FeSO4, CM+Fe). Cells were harvested and lysed, and β-galactosidase activity and cell protein were determined. The data are presented as specific activity, and the results are the mean of three separate experiments (± S.D.).