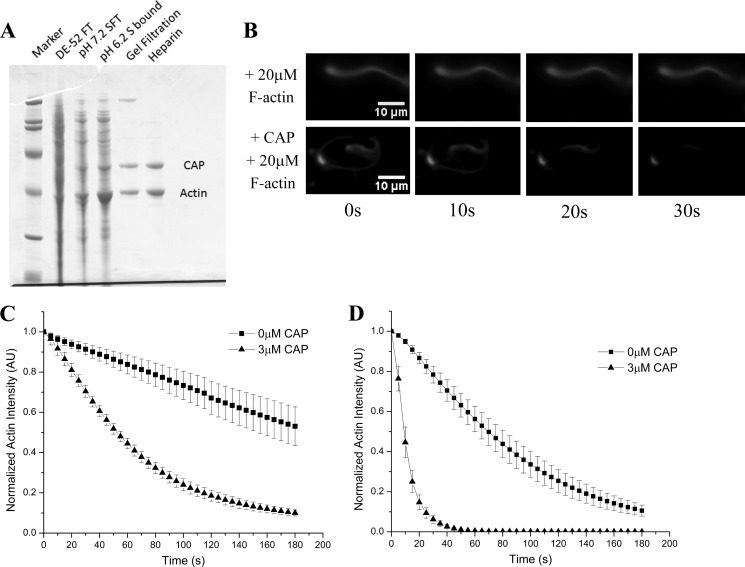
FIGURE 2.
CAP relieves F-actin-mediated inhibition of actin disassembly. A, SDS-PAGE summarizing purification of activity from bovine thymus extract. The activity was separated from all three known factors, first from coronin and AIP1 by flowing through DE52 beads and then from cofilin by flowing through an S column at physiological pH, thus indicating that this activity is a fourth actin disassembly factor. Mass spectrometry identified the lower band as actin, whereas the higher band was identified as CAP. B, excess F-actin inhibits the cofilin, coronin, and AIP1 mixture (top series), but recombinant CAP confers resistance to this inhibition (bottom series). C, quantification of CAP-mediated resistance to inhibition induced by excess (30 μm) F-actin. In the absence of CAP (squares), actin disassembly is greatly attenuated, but when CAP is added to the disassembly mixture (triangles), inhibition is lifted and efficient actin depolymerization is restored. D, in the absence of excess F-actin, CAP (triangles) markedly increases actin disassembly activity over the tripartite disassembly mixture alone (cofilin, coronin, and AIP1; squares). All experiments were done in the presence of cofilin (2 μm), coronin (2 μm), AIP1 (200 nm), and where noted CAP (3 μm). Displayed for each condition in C and D is the mean normalized actin fluorescence intensity resolved over time of at least three independent experiments ±S.D. Error bars represent S.D. AU, arbitrary units.