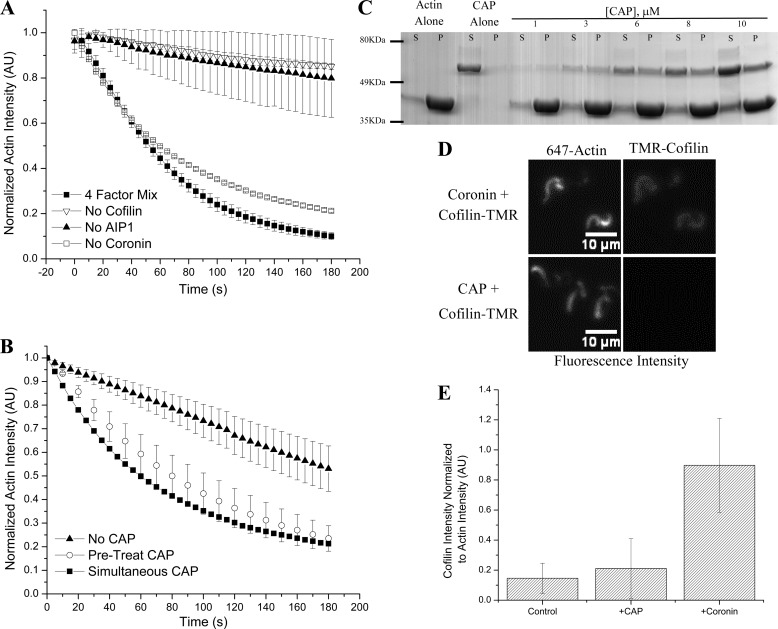
FIGURE 3.
CAP shows partial redundancy with coronin but remains mechanistically distinct. A, testing the four-factor actin disassembly mixture of cofilin, coronin, AIP1, and CAP for redundancy between CAP and each of the other factors in the presence of excess F-actin, we found that in the absence of coronin (open squares) activity was virtually unchanged (versus four-factor mixture; closed squares). Cofilin (inverted triangles) and AIP1 (closed triangles) remain necessary as their absence greatly attenuates actin depolymerization. B, CAP scores in a pretreatment assay (open circles) with activity similar to when CAP is added simultaneously with cofilin and AIP1 (squares). Triangles represent cofilin and AIP1 control. C, CAP, like coronin, directly binds F-actin as demonstrated in this Coomassie-stained SDS-PAGE gel showing supernatant (S) and pellet (P) fractions after sedimentation of 10 μm F-actin with an indicated amount of CAP. His6-CAP is the upper band (∼56 kDa), and actin is the lower band (43 kDa). D, unlike coronin (top series), CAP (bottom series) does not increase fluorescently labeled cofilin loading onto differentially labeled actin comet tails. E, quantification of cofilin loading. Although coronin significantly increases cofilin loading, CAP does not. Cofilin intensity is normalized to actin intensity in each image; bars represent the mean of 10 data points per condition ±S.D. All experiments except sedimentation utilized L. monocytogenese actin comet tails assembled as described. Kinetic data (A and B) are challenged with excess F-actin at 30 μm. Unless noted otherwise, 2 μm cofilin, 2 μm coronin, 2 μm CAP, and 200 nm AIP1 were used. Error bars represent S.D. TMR, tetramethylrhodamine; AU, arbitrary units.