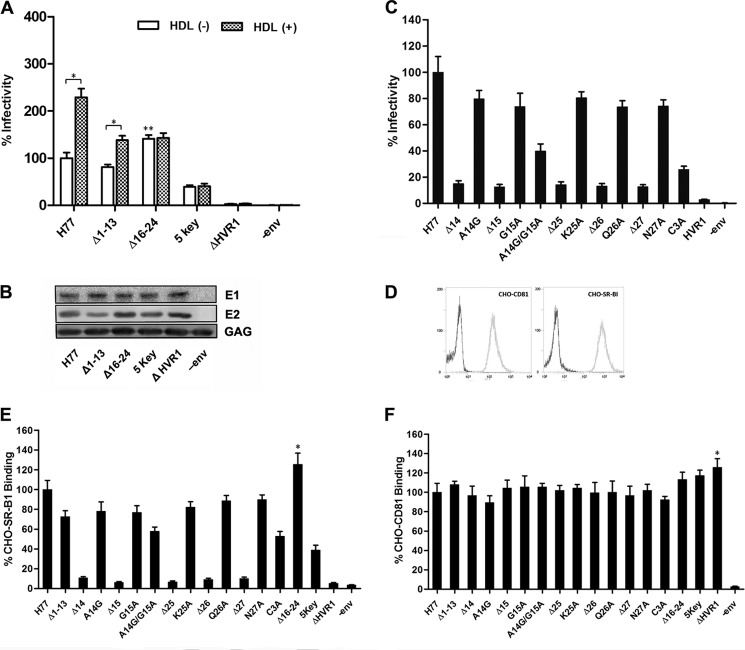
FIGURE 2.
Regions spanning aa 1–13 and 16–24 are dispensable for HCVpp cell entry. A, 293T cells were co-transfected with lentivirus transfer plasmid, HIV capsid plasmid, Rev plasmid, and plasmids encoding H77 envelope glycoproteins with deletion in HVR1. Pseudoparticles normalized to equal quantities of HCV E2 and HIV Gag proteins were used to infect Huh7.5 target cells, and infectivity was determined in the presence or absence of human HDL at a concentration of 6 μg/ml. The results are expressed as percentage of infectivity of wild-type H77. 5 key refers to envelope mutants preserving only aa 14, 15. and 25–27 in HVR1. Results are the means ± S.D. of four independent experiments. *, p < 0.001; **, p < 0.01 compared with H77 prototype pseudoparticles in the absence of HDL. B, pseudoparticles in culture media of 293T cells were pelleted through 20% sucrose cushions, and HCV envelope proteins and HIV capsid protein in pseudoparticle preparations were assayed by Western blotting. C, envelope expression plasmids containing various mutations were used to generate HCVpp, and the infectivity of the respective pseudoparticles was measured as described above. Co-transfection of 293T cells devoid of HCV envelope proteins (−env) plasmid was performed as a negative control. C3A represents a construct in which all three residues located at positions 25–27 were replaced with alanine. Data are means ± S.D. of three independent experiments. D, CHO cells were infected with lentivirus containing human SR-BI cDNA or CD81 cDNA or empty lentivirus, respectively, and the expression of human SR-BI and CD81 was determined by flow cytometry with mAb to CD81 or mouse polyclonal antibodies to SR-BI. CHO cells infected with empty lentivirus were used as a negative control. E, crude extracts of 293T cells transfected with HCV envelope protein expression plasmid were normalized by GNA capture ELISA, and the extracts containing equivalent amounts of prototype or mutant E2 protein were used to measure cell surface-associated SR-BI binding using a FACS-based assay. The extract of mock plasmid-transfected 293T cells that contained equivalent amounts of cellular protein as the E2 protein containing extract was used as a negative control (−env). The binding activity is expressed as the percentage of MFI relative to that of H77 prototype envelope proteins. Results are the means ± S.D. of three independent experiments. *, p < 0.05 compared with H77 prototype envelope proteins. F, binding of mutant E2 proteins to cell surface-associated CD81 was measured using a FACS-based assay. Results are the means ± S.D. of three independent experiments. *, p < 0.05 compared with H77 prototype envelope proteins.