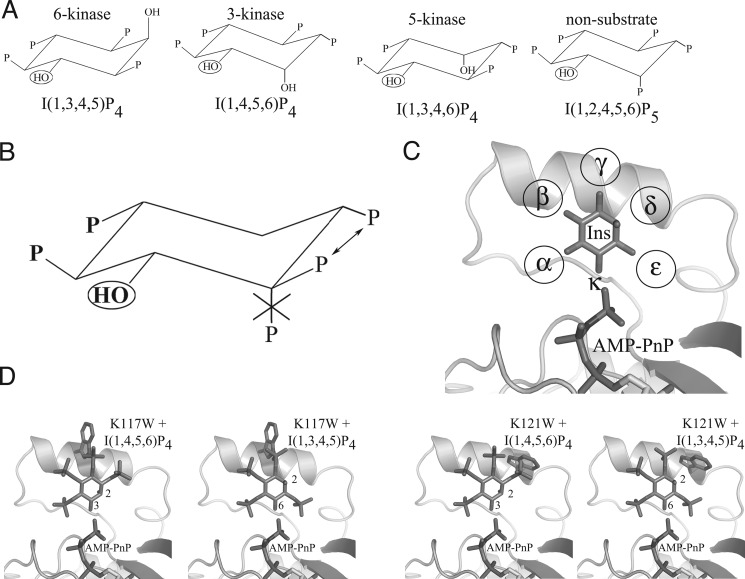
FIGURE 2.
Inositol phosphate species, binding motif, and A. thaliana IPMK selective designs. A, substrate and non-substrate IP species. Three A. thaliana IPMK IP substrate species, one for each kinase activity, and one non-substrate species are shown. All are orientated to preserve the position of the target hydroxyl (circled) and ring orientation. B, proposed IP substrate motif. Also shown are proposed rules for IP substrate selectivity by A. thaliana IPMK. Two phosphate groups are absolutely required (boldface type), the ring orientation must be maintained, one of two phosphate groups is required (arrows), and an axial phosphate cannot be tolerated if it occupies the first position clockwise of the target hydroxyl. C, phosphate binding pockets. Inositol in the active site of A. thaliana IPMK is shown conserving ring-carbon orientation. The phosphate pockets are designated α-ϵ clockwise from the kinase target hydroxyl κ. Surrounding residues are omitted for clarity. D, proposed binding of IP4 species with K117W and K121W mutants. Ins(1,3,4,5)P4 and Ins(1,4,5,6)P4 are modeled with either K117W or K121W mutant models. K117W is predicted to have steric clashes with both IP4 species, whereas K121W is predicted to clash with only Ins(1,4,5,6)P4.