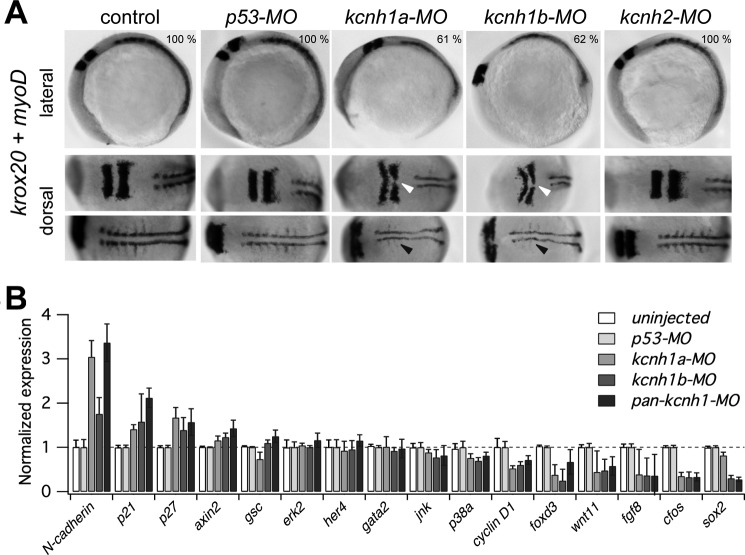
FIGURE 6.
Developmental delay of kcnh1 morphants affects hindbrain formation and somitogenesis. A, shown are representative whole-mount in situ hybridizations of zebrafish embryos with digoxigenin-labeled riboprobes for markers of hindbrain (krox20) and somites (myoD). Uninjected embryos (control) are compared with the indicated morphants. In lateral images (upper row), the dark-stained hindbrain rhombomeres 3 and 5 (krox20) and an extended stretch of myoD-stained somites are visible in the upper left and right region of the images, respectively. In the bottom row of the dorsal images the embryos are rotated to the left (anterior) by about 70 degrees. Note that in kcnh1a and kcnh1b morphants the rhombomeres 3 and 5 (white arrows) are thinner and that midline fusion of rhombomere 5 is incomplete. The lateral expansion and segmentation of somitic and presomitic mesoderm (black arrows) is reduced in both kcnh1 morphants. The percentage of embryos showing the given phenotype in two independent experiments is indicated in the upper images. B, early gene expression is modulated by kcnh1. Expression of the indicated genes in zebrafish morphants was analyzed by quantitative RT-PCR at 70% epiboly stage (7.5 hpf). Gene-specific quantitative PCR signals in uninjected controls as well as in kcnh1a, kcnh1b, and pan-kcnh1 morphants were normalized to p53-MO-injected controls, and ef1a served as the housekeeping gene for all samples. Genes are ordered according to the effect of pan-kcnh1-MO injection. Data are the mean ± S.D. from two independent experiments with each three reactions.