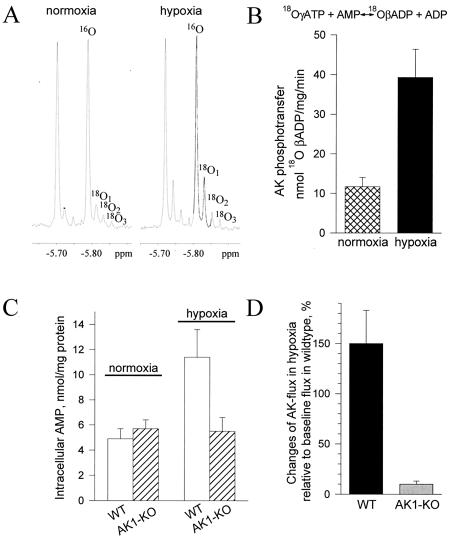
Figure 4.
AK phosphotransfer under metabolic stress. (A) 31P NMR spectra of guinea pig heart extracts under normoxic and hypoxic conditions. Incorporation of 18O into β-phosphoryls of ADP, which reflects the rate of AK-catalyzed phosphotransfer, induces the appearance of three 18O-labeled species (18O1, 18O2, and 18O3). Incorporation of 18O was significantly higher under hypoxia. (B) Average values for AK-catalyzed phosphotransfer flux in normoxia (n = 5; ▩) and hypoxia (n = 5; ■) in guinea pig heart determined by 18O/31P NMR spectroscopy. (C) AMP levels in wild-type (WT, n = 5; □) and AK1-KO (n = 5; ▨) mouse heart under normoxia and hypoxia determined by HPLC. (D) Blunted AK phosphotransfer response in the AK1-KO (n = 5; ░⃞) compared with the wild-type (n = 5; ■) mouse heart in hypoxia.