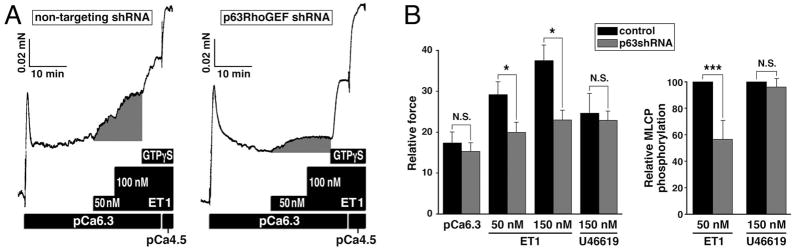
Fig. 3.
Inhibition of ET1-induced Ca2+-sensitized force and phosphorylation of MCLP (RhoA/ROCK substrate) when p63RhoGEF is partially silenced in mouse portal vein. A. ET1-, GTPγS- (i.e. maximal activation of RhoA/Ca2+-sensitized force) and Ca2+-induced force in permeabilized mouse portal veins following treatment with shRNA targeting p63RhoGEF or a non-targeting control. Note that ET1-induced Ca2+-sensitized force (shaded component) is inhibited by partial silencing of p63RhoGEF at constant Ca2+ concentration (pCa 6.3). GTPγS-induced force is a measure of the residual component of Ca2+-sensitization. pCa 4.5: maximal force. B. Summary of changes in ET1- (50 nM and subsequent addition of 100 nM; i.e. 150 nM final concentration) induced Ca2+-sensitized force as shown in panels A, in thromboxane analogue U46619- (150 nM) induced Ca2+-sensitized force, and in phosphorylation of the RhoA/ROCK substrate MLCP (i.e. phosphorylation of the MLCP subunit MYPT1 at Thr853) using the protocol shown in panel A. The reduction in force, normalized to maximal force induced by pCa 4.5, for 50 nM ET-1 (IC50 ) was 29.0±3.4 vs 19.7±2.6 respectively and at maximal ET-1 (150 nM) was 38.1±4.0% vs 23.0±2.5% respectively; no significant change in U46619-induced force between control and treated samples (mean values ± S.E. n = 12–14, * P < 0.01). Relative phosphorylations of MLCP: mean values ± S.E. n = 4, *** P < 0.02. From (Momotani et al. 2011).