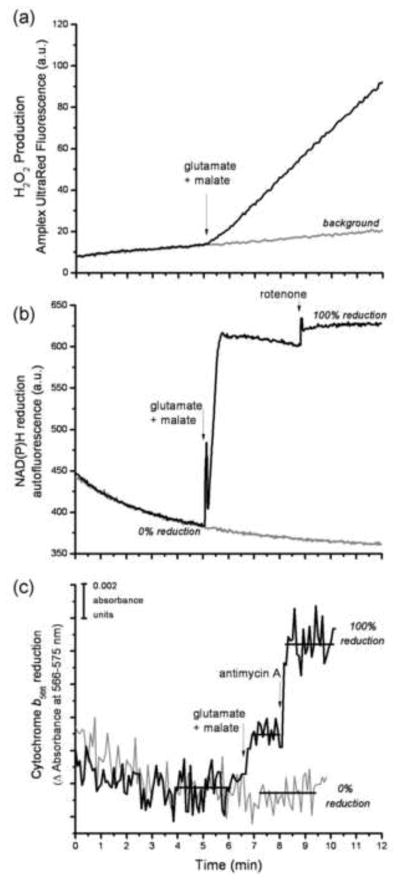
FIGURE 4. Design of the reporter-based superoxide assay.
The assay was designed to measure the rate of H2O2 production using Amplex UltraRed, and the steady-state reduction levels of NAD(P)H and cytochrome b566 under closely similar conditions. The timing of all additions was synchronized between the three assays. The addition of substrate, in this case 5 mM glutamate plus 5 mM malate, led to an increased rate of change of Amplex UltraRed fluorescence (a), and increased steady-state reduction levels of both NAD(P)H (b) and cytochrome b566 (c). The gray traces in each graph show the control in the absence of substrates or inhibitors. The 100% value for each reporter in (b) and (c) was established by addition of its relevant downstream inhibitor (4 μM rotenone or 2 μM antimycin A), as described in MATERIALS AND METHODS. The horizontal bars in (c) indicate the regions of data that were averaged to give the mean value used to calculate the % reduction of cytochrome b566. All of the data for the calibration curves (Figs 5 and 7) and measurements of native rates (Fig. 8) were collected in essentially this way.