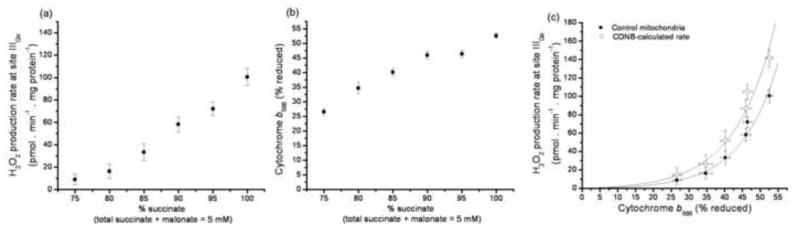
FIGURE 7. Relationship between the rate of superoxide production by site IIIQo and the reduction state of cytochrome b566.
(a) Dependence of the rate of myxothiazol-sensitive H2O2 production on succinate concentration at fixed succinate+malonate concentration in the presence of rotenone. Data were corrected for the contribution of site IF (Fig. 6c and Fig. 5c). (b) Dependence of cytochrome b566 reduction on succinate concentration in parallel incubations (100% reduction was subsequently established by addition of 2 μM Antimycin A). (c) Final calibration of the relationship between the rate of superoxide production from site IIIQo and cytochrome b566 reduction state, obtained by combining panels (a) and (b). Filled symbols: control mitochondria; open symbols: control values after correction to CDNB-treated mitochondria using Eq. 1, assuming that 50% of superoxide from site IIIQo was produced in the matrix (see [26]). Where not visible, error bars are contained within the points. Lines show exponential relationships (for simplicity), fitted by non-linear regression to give the parameter values in Eq. 3. See MATERIALS AND METHODS. Data are means ± SEM (n = 9).