Abstract
Prostate stem cell antigen (PSCA) is a glycosylphosphatidylinositol-anchored cell surface antigen with an organ-dependent expression pattern in cancers; e.g., up-regulated in prostate cancer and down-regulated in gastric cancer. Previously it was reported that PSCA is not expressed in the normal pancreas but aberrantly expressed in pancreatic cancer. In this present study, we identified PSCA expression in islets of the pancreas by immunohistochemistry, which was co-localized with four islet-cell markers: insulin, glucagon, somatostatin and pancreatic polypeptide. In our investigation of the transcription start site of PSCA, we found a non-coding splicing variant of PSCA as well as authentic PSCA transcripts in mRNA samples from a normal pancreas. Both the transcripts were also identified in several pancreatic cancer cell lines. We previously reported that PSCA expression is correlated to the methylation status of the enhancer region in gastric and gallbladder cancer cell lines but not in pancreatic cancer cell lines, suggesting that PSCA expression is regulated in a diff erent mode in pancreatic cancer from that in gastric and gallbladder cancers.
Keywords: GPI-anchored protein, Islet cells, Pancreatic cancer, Splicing variant, Immunohistochemistry
Introduction
The prostate stem cell antigen (PSCA) gene encodes a glycosylphosphatidylinositol (GPI)-anchored membrane protein with an unknown biological function [1]. As with other GPI-anchored proteins, it is thought that PSCA locates in a lipid raft on the outer surface of the cell membrane, a special microdomain enriched in glycosphingolipids, cholesterol and other lipidated proteins, and has some functional involvement in subcellular signal transduction [2]. PSCA was originally identified as a gene up-regulated in prostate cancer [3]. It is also up-regulated in other tumors including urinary bladder cancer, renal cell carcinoma, hydatidiform mole and ovarian mucinous tumor, where PSCA is thought to abet tumor progression [1]. In contrast, down-regulation of the gene was reported in gastric and gallbladder cancers, where it may act as a tumor suppressor [4, 5]. Moreover, there is another type of expression pattern: no expression in normal tissues but expressed in malignant counterparts, which is seen in pancreatic cancer [6], non-small cell lung cancer [7] and glioma [8]. It was reported that PSCA expression was not observed in the normal pancreas by northern blotting or immunohistochemistry and that its transcript could be a practical biomarker in detecting pancreatic cancer [6]. In this study, we identified PSCA expression in islets of the normal pancreas, which harbor endocrine cells, by immunohistochemistry using a mouse monoclonal anti-PSCA antibody.
Materials and Methods
Immunohistochemistry
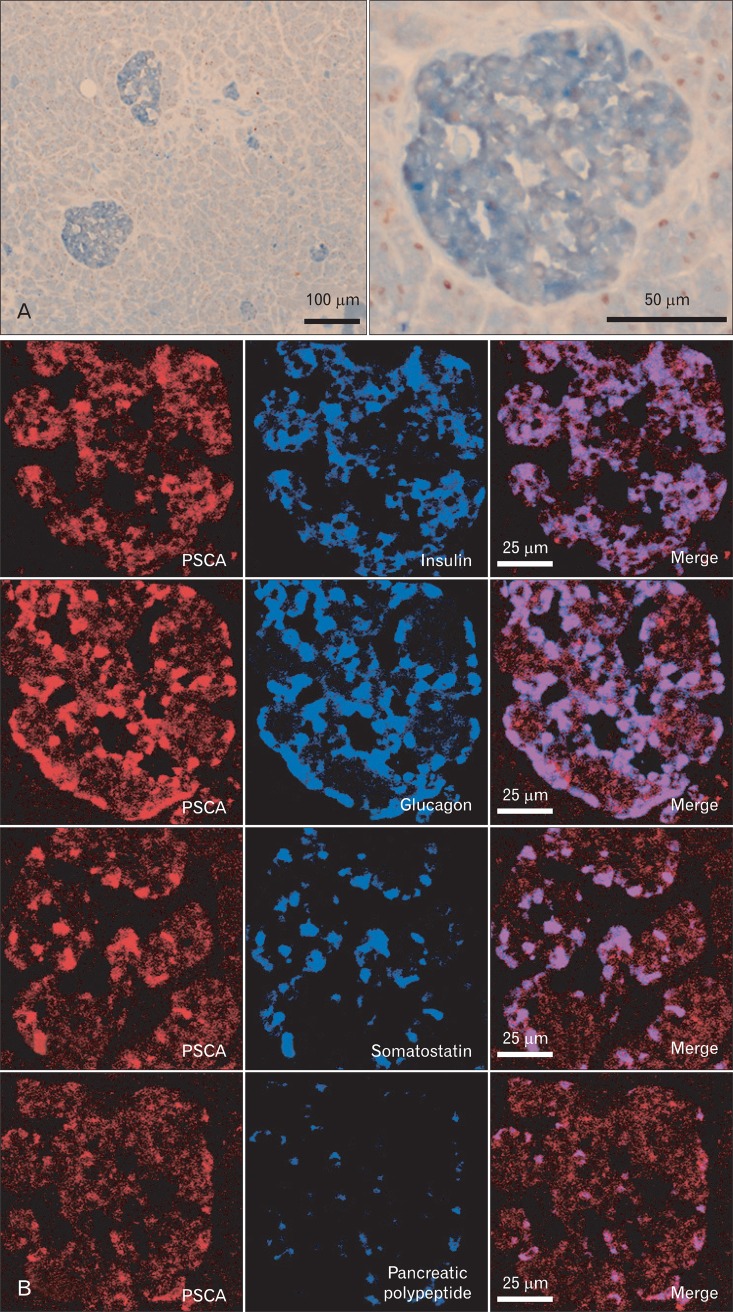
Embedded normal pancreas specimens on glass were purchased from BioChain Institute (Newark, CA, USA). Immunohistochemistry (Fig. 1A) was performed by the same procedure as described in the previous studies [4, 5] with mouse monoclonal anti-PSCA antibody whose specificity had been proved previously [4], rabbit anti-proliferating cell nuclear antigen (PCNA) antibody (sc-7907, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sections were incubated at 4℃ overnight with both antibodies simultaneously, and then with alkaline phosphatase conjugated anti-mouse IgG antibody and peroxidase conjugated anti-rabbit IgG antibody (NICHIREI Biosciences Inc., Tokyo, Japan) for an hour at room temperature. PSCA protein was visualized by Alkaline Phosphatase Substrate Kit III (Vector Laboratories, Burlingame, CA, USA) and PCNA by Vector NovaRED Substrate Kit for Peroxidase (Vector Laboratories). The fluorescent immunohistochemistry (Fig. 1B) was conducted using anti-PSCA, rabbit anti-insulin (sc-9186, Santa Cruz Biotechnology), rabbit anti-glucagon (ab18461, Abcam Japan, Tokyo, Japan), rabbit anti-somatostatin (A0566, Dako Japan, Tokyo, Japan) and goat anti-pancreatic polypeptide (NB100-1793, Novus Biologicals, Littleton, CO, USA) antibodies for primary antibodies, and anti-mouse IgG antibody-Rhodamin, anti-rabbit IgG-FITC and anti-goat IgG-FITC conjugates (sc-2300, sc-2090, sc-2024, respectively, Santa Cruz Biotechnology) for secondary antibodies. The fluorescent signals were obtained by Zeiss LSM510 META Confocal Imaging System (Carl Zeiss MicroImaging, LLC, Thornwood, NY, USA).
Fig. 1.
Prostate stem cell antigen (PSCA) is expressed in the islet of human normal pancreas (immunohistochemistry). (A) Double staining with anti-PSCA (blue) and anti-proliferating cell nuclear (brown) antibodies. The right panel is magnification of the islet shown in the left column. (B) Fluorescent double staining of anti-PSCA antibody (red) and four islet-cell markers (blue), insulin (β cell), glucagon (α cell), somatostatin (δ cell) or pancreatic polypeptide (PP cell). Each series of the image was obtained by focusing on the islet-cell markers.
Cell lines
The cell lines analysed in this study were described elsewhere; pancreatic cancer cell lines [6], gastric cancer cell lines [4], and gallbladder cancer cell lines [5].
RNA ligase-mediated rapid amplification of 5' cDNA end (RLM-5' RACE)
The RLM-5' RACE was conducted on commercially available total RNA isolated from normal human pancreas (BioChain, Newark, CA, USA), and on total RNA isolated from the pancreatic cancer cell lines, using a GeneRacer kit (Invitrogen, Tokyo, Japan). A primer for the 1st strand synthesis (gene specific primer 1) was designed on the sequence in the 2nd exon, and nested PCR was performed with two sets of primers; a GeneRacer 5' primer and the gene specific primer 1, and a GeneRacer 5' nested primer and a primer (gene specific primer 2) containing a sequence partially overlapped with the gene specific primer 1 (Table 1). The PCR products were cloned into pCR4-TOPO vector (Invitrogen) and sequenced.
Table 1.
Primers
RLM-5' RACE, RNA ligase-mediated rapid amplification of 5' cDNA end; RT-PCR, reverse transcription polymerase chain reaction; PSCA, prostate stem cell antigen; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
The templates were synthesized from commercially available total RNA isolated from normal human pancreas, stomach and gallbladder (BioChain), or total RNA isolated from the pancreatic, gastric and bladder cancer cell lines, using the ThermoScript RT-PCR System (Invitrogen). Quantitative RT-PCR in Figs. 2A and 3A was performed by TaqMan Gene Expression Assay (Life Technologies Japan, Tokyo, Japan; Applied Biosystems assay ID: Hs00194665_m1 for PSCA, Applied Biosystems Part No. 4326317E for glyceraldehyde-3-phosphate dehydrogenase [GAPDH]), which was conducted for 40 cycles under a condition of 2 steps of temperature: 95℃ for 15 seconds and 60℃ for 60 seconds, by ABI PRISM 7900HT Sequence Detection System (Life Technologies Japan). Quantitative RT-PCR in Fig. 2C was performed with gene expression assay using SYBR Premix Ex Taq II (Takara Bio Inc., Shiga, Japan), conducted in 40 cycles under a condition of 2 steps of temperature: 95℃ for 5 seconds and 60℃ for 30 seconds, by the ABI PRISM 7900HT Sequence Detection System. The relative transcript level was calculated using the Ct value of GAPDH transcript as reference.
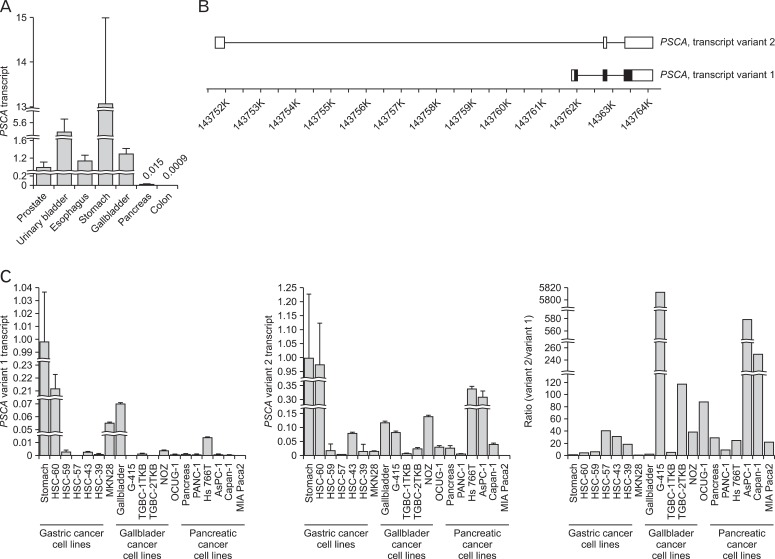
Fig. 2.
Two splicing variants of the prostate stem cell antigen (PSCA) transcript are expressed in normal pancreatic, gastric and gallbladder tissues and in cancer cell lines derived from their cancers. (A) Quantitative reverse transcription polymerase chain reaction (RT-PCR) revealed PSCA transcript (variant 1) in human normal pancreas. Colon is negative control as PSCA is not expressed in that organ. (B) Schematic representation of the structure of the two variants. Position in chromosome 8q24 is based on NCBI Build 37.3. (C) Quantitative RT-PCR revealed expression of variants 1 and 2 in the normal tissues and the cancer cell lines, and variant 2 is dominant in the pancreas and pancreatic cancer cell lines.
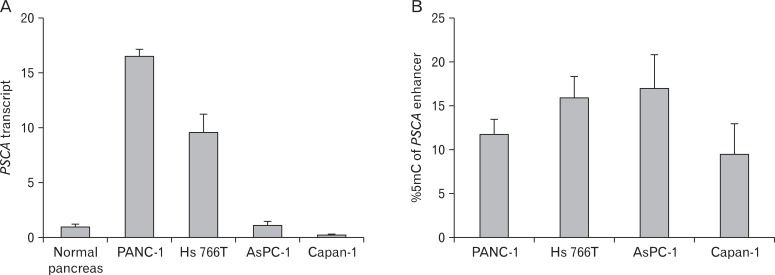
Fig. 3.
Bisulfite-pyrosequencing revealed that the DNA methylation status of the prostate stem cell antigen (PSCA) enhancer (B) is not correlated to expression level (A) in the pancreatic cancer cell lines.
DNA methylation analysis
The DNA methylation status of the PSCA enhancer in the pancreatic cancer cell lines was analysed by bisulfite-Pyrosequencing procedure in the same manner as described in the previous report [5]. DNA samples were isolated from the pancreatic cancer cell lines with FlexiGene DNA Kit (Qiagen, Tokyo, Japan) and treated with EpiTect Bisulfite Kit (Qiagen) according to the manufacturer's instructions. DNA methylation of the PSCA gene (GenBank accession No. NG_011722) was investigated by bisulfite-Pyrosequencing at PSCA enhancer region (position, -3,090 to -2,880) which is in the PSCA enhancer region located at -2.7 to -3 kb from the transcription initiation site [5]. PCR was performed in triplicate under a condition of 45 cycles of 3 steps of temperature, 95℃ for 1 minute, 50℃ for 1 minute, and 72℃ for 1 minute, after incubation at 95℃ for 15 minutes with HotStarTaq (Qiagen). Biotinylated PCR product was purified and proceeded to the Pyrosequencing reaction, which was performed using the Pyrosequencing 96 Sample Prep Tool (Qiagen) and PSQ96MA (Biotage, Uppsala, Sweden). The data of the methylation status for the CpG site in bisulfite-treated sequences were obtained using the primers shown in Table 1, and the average frequency of methylated CpG sites was used as the methylation status index (%5mC).
Results
It was previously reported that PSCA was not expressed in the normal pancreas, but we identified PSCA expression in the islets of the normal human pancreas by immunohistochemistry, and double-staining for 4 islet-cell markers revealed that PSCA is expressed in all of the 4 islet cells: α, β, δ, and PP cells (Fig. 1). Pancreatic expression was confirmed by quantitative RT-PCR (Fig. 2A). To identify the regulatory region for PSCA expression in the pancreas, we first performed RLM-5' RACE to identify the transcription start site (TSS) of the PSCA transcripts, which revealed two splicing variants in the pancreas: one is the authentic transcript (variant 1, NCBI accession No. NM_005672) generally detected in other tissues; the other is a non-coding type (variant 2, NR_033343) transcribed at the TSS about 1 kb upstream of that of the authentic one, suggesting that the regulatory region of variant 2 is different from that of the authentic (Fig. 2B). As the amount of variant 2 was small, it may be expressed in the islets. We also examined the expression of variant 2 in the normal stomach and gallbladder and in cell lines derived from their cancers, in addition to pancreatic cancer cell lines, by RT-PCR and found the transcript in all the normal tissues and the cell lines except MIA Paca2 (Fig. 2C). Variant 2 expression was dominant over variant 1 expression in the normal pancreas and the pancreatic cancer cell lines, although it codes no protein. We previously reported that variant 1 expression in gastric and gallbladder cancer cell lines is associated with the DNA methylation level in the enhancer region: the cell lines with relatively high PSCA expression tend to have a lower methylation level [5], but the relation was not observed in pancreatic cancer cell lines (Fig. 3).
Discussion
Although its function is unknown, in the normal prostate epithelium, PSCA is expressed in late intermediate cells in the differentiation process but not in mature secretary cells [9]. In the stomach, PSCA is mainly expressed in the epithelium of the middle portion of the gastric gland, which harbors a pre-pit cell, a transit amplifying precursor of a mucous-secreting pit cell [5]. In these organs, PSCA seems to have a role in the cells differentiating, rather than in differentiated mature cells. It was reported that pancreatic islets contain multipotent stem cells and that the islet cells continue to proliferate slowly for turning over [10, 11]. In this sense, the islet cells possess a property of immature cells, and PSCA may have a function related to the differentiation and/or proliferation of the cells.
In this study, we performed immunohistochemistry on the normal human pancreas, which revealed PSCA expression in islet cells. The expression was confirmed by RT-PCR and, in the process of identification of the TSS in the pancreas, we observed two types of PSCA transcript in the normal pancreas as well as in pancreatic cancer cell lines. It is important to note the fact that PSCA is expressed in the normal pancreas, because there are several reports suggesting that PSCA can be utilized as a biomarker of pancreatic cancer [12-14] and it is considered as a target molecule in the treatment of pancreatic and prostate cancers [15, 16]. Clinical applications targeting PSCA have a potential risk of leading to misdiagnosis and adverse events in medical practice.
Acknowledgements
This study was supported by a research grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (JST grant) and by a Grants-in-Aid for Scientific Research (KAKENHI) by the Japan Society for the Promotion of Science (No. 23501327).
References
- 1.Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer Res. 2010;16:3533–3538. doi: 10.1158/1078-0432.CCR-09-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharom FJ, Radeva G. GPI-anchored protein cleavage in the regulation of transmembrane signals. Subcell Biochem. 2004;37:285–315. doi: 10.1007/978-1-4757-5806-1_9. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, Loda M, Witte ON. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Study Group of Millennium Genome Project for Cancer. Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F, Kato S, Matsukura N, Matsuda N, Nakamura T, Hyodo I, Nishina T, Yasui W, Hirose H, Hayashi M, Toshiro E, Ohnami S, Sekine A, Sato Y, Totsuka H, Ando M, Takemura R, Takahashi Y, Ohdaira M, Aoki K, Honmyo I, Chiku S, Aoyagi K, Sasaki H, Yanagihara K, Yoon KA, Kook MC, Lee YS, Park SR, Kim CG, Choi IJ, Yoshida T, Nakamura Y, Hirohashi S. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730–740. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 5.Ono H, Hiraoka N, Lee YS, Woo SM, Lee WJ, Choi IJ, Saito A, Yanagihara K, Kanai Y, Ohnami S, Chiwaki F, Sasaki H, Sakamoto H, Yoshida T, Saeki N. Prostate stem cell antigen, a presumable organ-dependent tumor suppressor gene, is down-regulated in gallbladder carcinogenesis. Genes Chromosomes Cancer. 2012;51:30–41. doi: 10.1002/gcc.20928. [DOI] [PubMed] [Google Scholar]
- 6.Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SR, Leach SD, Ryu B, Skinner HG, Goggins M, Jaffee EM, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- 7.Kawaguchi T, Sho M, Tojo T, Yamato I, Nomi T, Hotta K, Hamada K, Suzaki Y, Sugiura S, Kushibe K, Nakajima Y, Taniguchi S. Clinical significance of prostate stem cell antigen expression in non-small cell lung cancer. Jpn J Clin Oncol. 2010;40:319–326. doi: 10.1093/jjco/hyp181. [DOI] [PubMed] [Google Scholar]
- 8.Geiger KD, Hendruschk S, Rieber EP, Morgenroth A, Weigle B, Juratli T, Senner V, Schackert G, Temme A. The prostate stem cell antigen represents a novel glioma-associated antigen. Oncol Rep. 2011;26:13–21. doi: 10.3892/or.2011.1265. [DOI] [PubMed] [Google Scholar]
- 9.Tran CP, Lin C, Yamashiro J, Reiter RE. Prostate stem cell antigen is a marker of late intermediate prostate epithelial cells. Mol Cancer Res. 2002;1:113–121. [PubMed] [Google Scholar]
- 10.Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE, Wheeler MB, van der. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281–293. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development. 2009;136:3567–3574. doi: 10.1242/dev.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grubbs EG, Abdel-Wahab Z, Tyler DS, Pruitt SK. Utilizing quantitative polymerase chain reaction to evaluate prostate stem cell antigen as a tumor marker in pancreatic cancer. Ann Surg Oncol. 2006;13:1645–1654. doi: 10.1245/s10434-006-9029-5. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy DM, Maitra A, Argani P, Rader AE, Faigel DO, Van Heek NT, Hruban RH, Wilentz RE. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: mesothelin and prostate stem cell antigen labeling increases accuracy in cytologically borderline cases. Appl Immunohistochem Mol Morphol. 2003;11:238–243. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Komatsu N, Terakawa N, Yanagimoto Y, Oka M, Sasada T, Mine T, Gouhara S, Shichijo S, Okuda S, Itoh K. Increased levels of IgG antibodies against peptides of the prostate stem cell antigen in the plasma of pancreatic cancer patients. Oncol Rep. 2007;18:161–166. [PubMed] [Google Scholar]
- 15.Wente MN, Jain A, Kono E, Berberat PO, Giese T, Reber HA, Friess H, Büchler MW, Reiter RE, Hines OJ. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas. 2005;31:119–125. doi: 10.1097/01.mpa.0000173459.81193.4d. [DOI] [PubMed] [Google Scholar]
- 16.Morris MJ, Eisenberger MA, Pili R, Denmeade SR, Rathkopf D, Slovin SF, Farrelly J, Chudow JJ, Vincent M, Scher HI, Carducci MA. A phase I/IIA study of AGS-PSCA for castration-resistant prostate cancer. Ann Oncol. 2012 May 02; doi: 10.1093/annonc/mds078. [Epub]. http://dx.doi.org/10.1093/annonc/mds078. [DOI] [PMC free article] [PubMed] [Google Scholar]