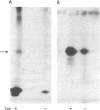
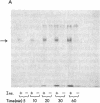
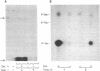
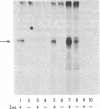
Abstract
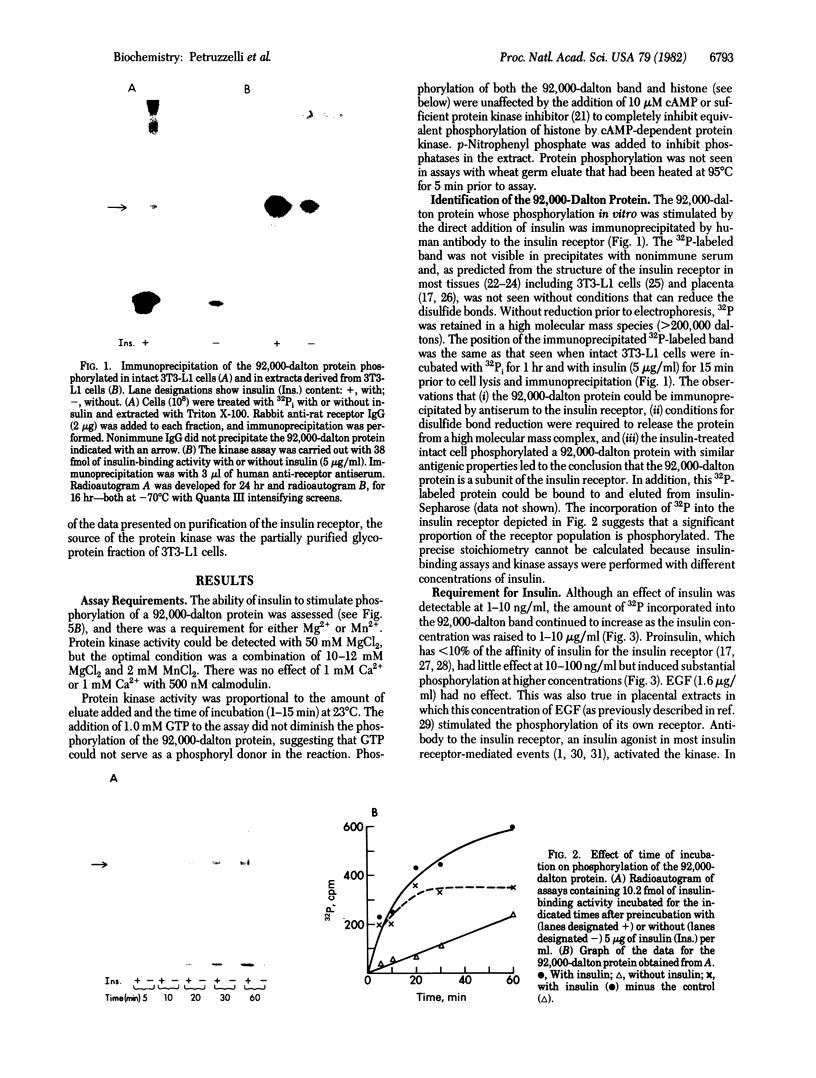
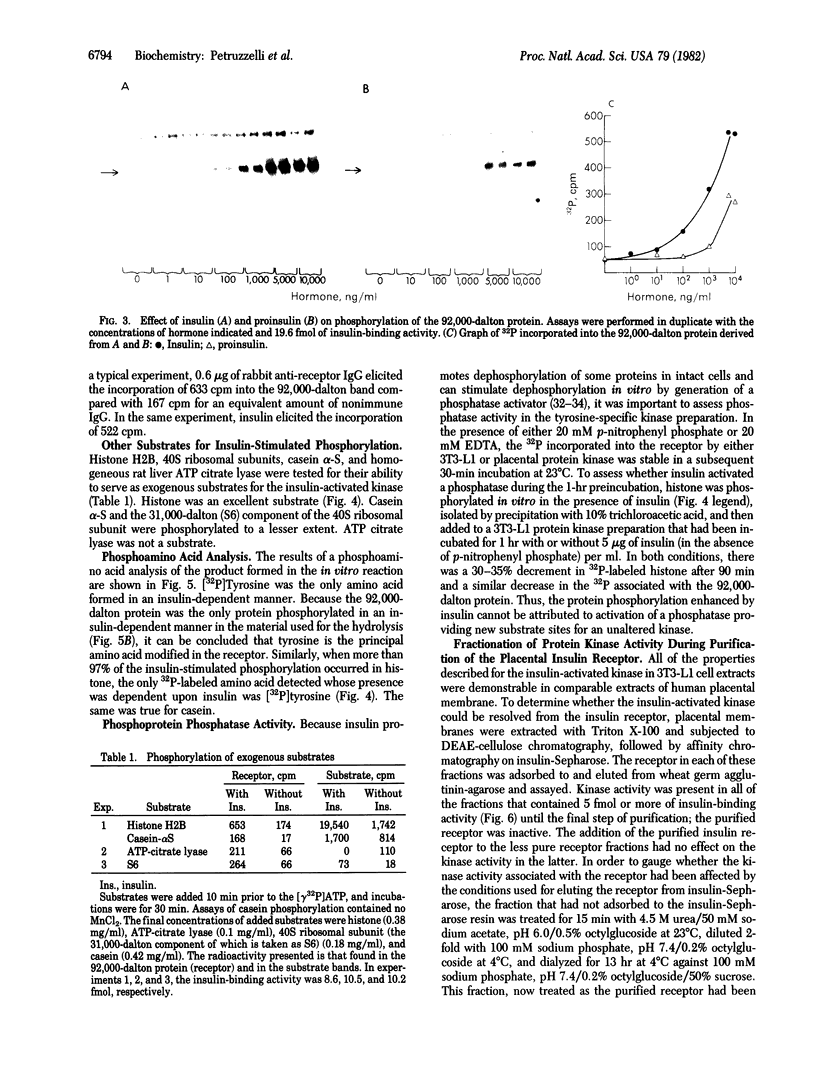
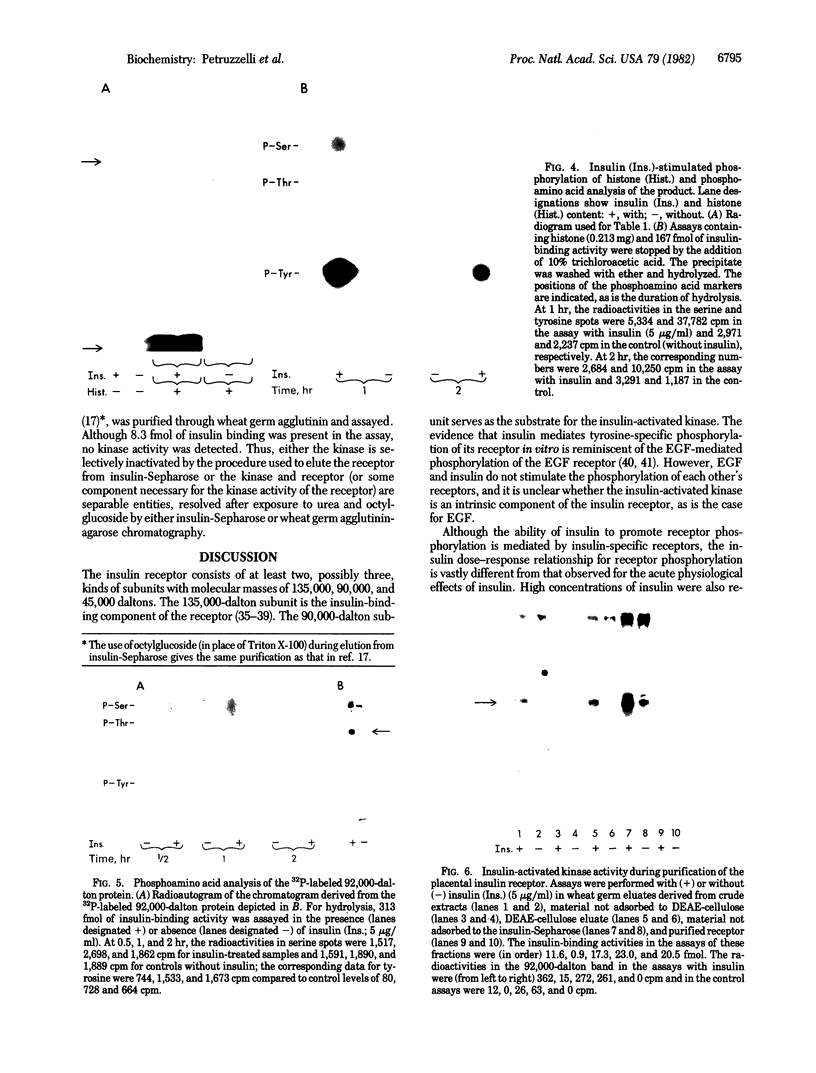
Insulin activates a tyrosine-specific cAMP-independent protein kinase when added directly to detergent extracts of differentiated 3T3-L1 adipocytes and humal placental membranes. The kinase is also activated by antibody to the insulin receptor and, to a lesser extent, by proinsulin. It catalyzes the phosphorylation of the 92,000-dalton component of the insulin receptor, histone, and casein; in each case, tyrosine is the principal amino acid modified. Under the conditions used to activate the kinase, insulin does not affect the rate of dephosphorylation of the receptor or of histone. The insulin-activated kinase is copurified with the human placental insulin receptor until the final elution from insulin-Sepharose. It remains to be established whether the kinase and the insulin receptor are separate molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. C., Kowaloff E. M., Witters L. A., Dennihy D. T., Avruch J. Purification of a hepatic 123,000-dalton hormone-stimulated 32P-peptide and its identification as ATP-citrate lyase. J Biol Chem. 1979 Aug 25;254(16):8052–8056. [PubMed] [Google Scholar]
- Avruch J., Leone G. R., Martin D. B. Effects of epinephrine and insulin on phosphopeptide metabolism in adipocytes. J Biol Chem. 1976 Mar 10;251(5):1511–1515. [PubMed] [Google Scholar]
- Benjamin W. B., Singer I. Actions of insulin, epinephrine, and dibutyryl cyclic adenosine 5'-monophosphate on fat cell protein phosphorylations. Cyclic adenosine 5'-monophosphate dependent and independent mechanisms. Biochemistry. 1975 Jul 29;14(15):3301–3309. doi: 10.1021/bi00686a003. [DOI] [PubMed] [Google Scholar]
- Benjamin W. B., Singer I. Effect of insulin on the phosphorylation of adipose tissue protein. Biochim Biophys Acta. 1974 May 10;351(1):28–41. doi: 10.1016/0005-2795(74)90063-4. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Poliner L., King L., Jr Protein phosphorylation in human placenta. Stimulation by epidermal growth factor. Mol Cell Endocrinol. 1980 Jun;18(3):189–199. doi: 10.1016/0303-7207(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Rosen O. M., Rubin C. S. Identification and characterization of a latent pool of insulin receptors in 3T3-L1 adipocytes. J Biol Chem. 1982 May 25;257(10):5350–5358. [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Shechter Y., Bissell K., Cuatrecasas P. Purification and properties of insulin receptors from rat liver membranes. Biochem Biophys Res Commun. 1977 Aug 8;77(3):981–988. doi: 10.1016/s0006-291x(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Jarett L., Seals J. R. Pyruvate dehydrogenase activation in adipocyte mitochondria by an insulin-generated mediator from muscle. Science. 1979 Dec 21;206(4425):1407–1408. doi: 10.1126/science.505013. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K., Filier J. S., Jarrett D. B. Effects of autoantibodies to the insulin receptor on isolated adipocytes. Studies of insulin binding and insulin action. J Clin Invest. 1977 Nov;60(5):1094–1106. doi: 10.1172/JCI108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Kahn C. R., Hedo J. A., Van Obberghen E., Yamada K. M. Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6917–6921. doi: 10.1073/pnas.78.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larner J., Galasko G., Cheng K., DePaoli-Roach A. A., Huang L., Daggy P., Kellogg J. Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science. 1979 Dec 21;206(4425):1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Insulin trigger, cyclic AMP-dependent activation and phosphorylation of a plasma membrane cyclic AMP phosphodiesterase. Nature. 1980 Aug 28;286(5776):904–906. doi: 10.1038/286904a0. [DOI] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Fat cell protein phosphorylation. Identification of phosphoprotein-2 as ATP-citrate lyase. J Biol Chem. 1979 Sep 25;254(18):9232–9236. [PubMed] [Google Scholar]
- Rosen O. M., Rubin C. S., Cobb M. H., Smith C. J. Insulin stimulates the phosphorylation of ribosomal protein S6 in a cell-free system derived from 3T3-L1 adipocytes. J Biol Chem. 1981 Apr 25;256(8):3630–3633. [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Seals J. R., Czech M. P. Characterization of a pyruvate dehydrogenase activator released by adipocyte plasma membranes in response to insulin. J Biol Chem. 1981 Mar 25;256(6):2894–2899. [PubMed] [Google Scholar]
- Siegel T. W., Ganguly S., Jacobs S., Rosen O. M., Rubin C. S. Purification and properties of the human placental insulin receptor. J Biol Chem. 1981 Sep 10;256(17):9266–9273. [PubMed] [Google Scholar]
- Smith C. J., Rubin C. S., Rosen O. M. Insulin-treated 3T3-L1 adipocytes and cell-free extracts derived from them incorporate 32P into ribosomal protein S6. Proc Natl Acad Sci U S A. 1980 May;77(5):2641–2645. doi: 10.1073/pnas.77.5.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Wejksnora P. J., Warner J. R., Rubin C. S., Rosen O. M. Insulin-stimulated protein phosphorylation in 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2725–2729. doi: 10.1073/pnas.76.6.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speaker M. G., Sturgill T. W., Orlow S. J., Chia G. H., Pifko-Hirst S., Rosen O. M. The effects of trifluoperazine on the macrophage-like cell line, J774. Ann N Y Acad Sci. 1980;356:162–178. doi: 10.1111/j.1749-6632.1980.tb29609.x. [DOI] [PubMed] [Google Scholar]
- Swergold G. D., Rosen O. M., Rubin C. S. Hormonal regulation of the phosphorylation of ATP citrate lyase in 3T3-L1 adipocytes. Effects of insulin and isoproterenol. J Biol Chem. 1982 Apr 25;257(8):4207–4215. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Wisher M. H., Baron M. D., Jones R. H., Sönksen P. H. Photoreactive insulin analogues used to characterise the insulin receptor. Biochem Biophys Res Commun. 1980 Jan 29;92(2):492–498. doi: 10.1016/0006-291x(80)90360-5. [DOI] [PubMed] [Google Scholar]
- Yeung C. W., Moule M. L., Yip C. C. Photoaffinity labeling of insulin receptor with an insulin analogue selectively modified at the amino terminal of the B chain. Biochemistry. 1980 May 13;19(10):2196–2203. doi: 10.1021/bi00551a031. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ochoa S. Purification of eukaryotic initiation factor 1 (EIF1) from Artemia salina embryos. Methods Enzymol. 1974;30:197–206. doi: 10.1016/0076-6879(74)30022-5. [DOI] [PubMed] [Google Scholar]