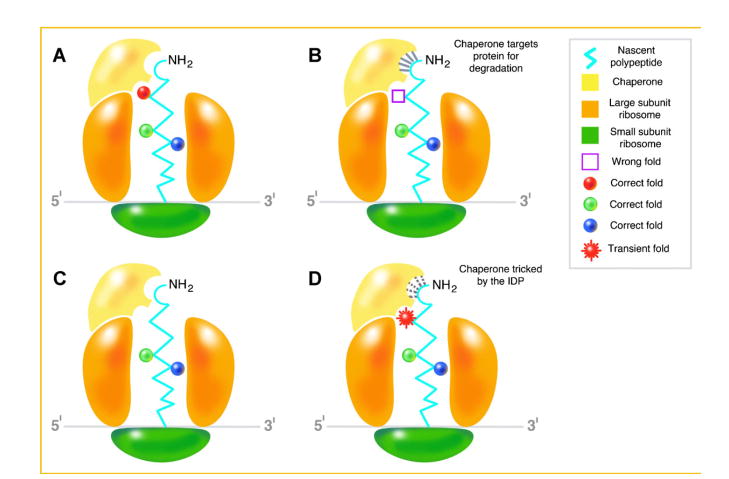
Fig. 1.
Coupled folding and binding and the order/disorder paradox. A: The nascent polypeptide encoding a highly ordered globular protein begins folding as it traverses the exit channel of the large subunit of the ribosome. It is “recognized” by the chaperone as properly folded. B: The polypeptide is misfolded and therefore, is identified by the chaperone for degradation. The interaction between the misfolded polypeptide and chaperone is indicated by the solid lines near the NH2 terminal. C: The nascent polypeptide encoding an IDP begins folding as it traverses the exit channel of the large subunit of the ribosome. D: Therefore, it is “recognized” by the chaperone as misfolded/damaged and the chaperone binds to it as indicated by the dotted lines. However, by virtue of its innate ability to transition from disorder to order upon binding, the IDP assumes a “transient” fold that “tricks” the chaperone into believing that it is indeed ordered and folded to evade degradation.