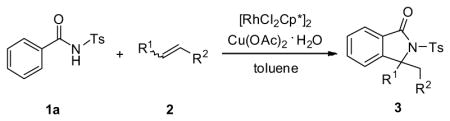
Table 2.
Substrate scope of various alkenes.a
| |||
|---|---|---|---|
| Entry | Alkene | Product | Yield (%)b |
| 1 |
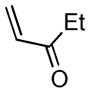 2b |
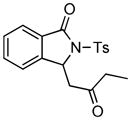 3g |
78 |
| 2 |
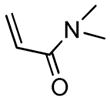 2c |
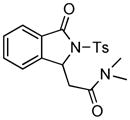 3h |
82 |
| 3 |
2d |
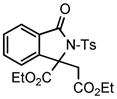 3i |
82 |
| 4 |
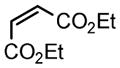 2e |
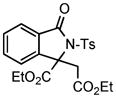 3i |
80 |
| 5 |
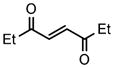 2f |
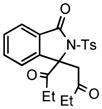 3j |
51 |
| 6 |
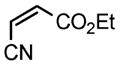 2g |
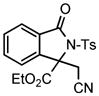 3k |
66 |
| 7c |
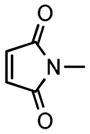 2h |
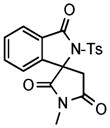 3l |
73 |
Reaction conditions: 1a (0.10 mmol), 2 (0.12 mmol), [RhCl2Cp*]2 (0.004 mmol) and Cu(OAc)2·H2O (0.20 mmol) in toluene, 130 °C for 24 h.
Isolated yield.
48 h.
