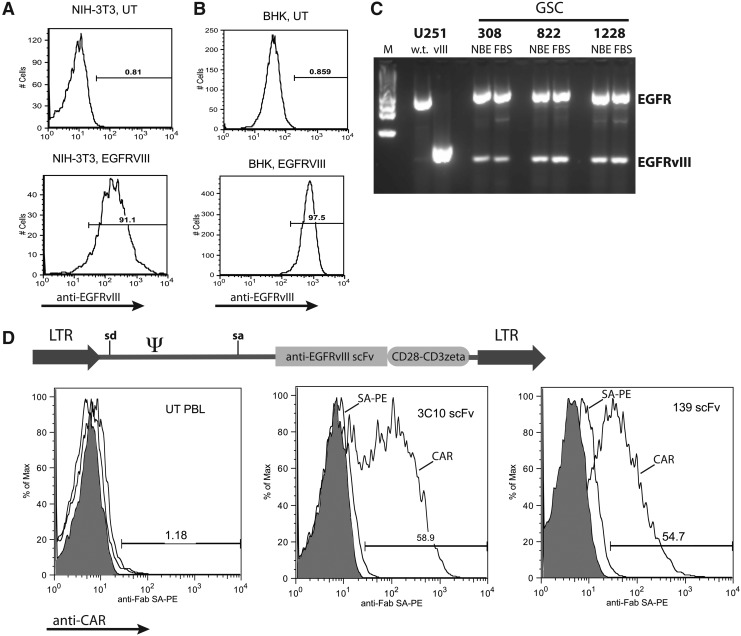
FIG. 1.
Development of CARs targeting EGFRvIII. NIH-3T3 cells (A) and BHK cells (B) were transduced with a retroviral vector expressing EGFRvIII and a neomycin resistance gene. Following selection in neomycin analogue G418, cells were subject to FACS analysis with anti-EGFRvIII mAb as shown (UT, untransduced). Histograms are representative of three independent determinations. (C) GSC lines were cultured in Neurobasal medium supplemented with bFGF and EGF (NBE) or in RPMI-1640 supplemented with 10% FBS for 3 days (to induce differentiation). RNA was extracted from each cell population and subject to RT-PCR using primers that span the variant III deletion in the wild-type EGFR gene, followed by gel electrophoresis of the products (M, marker). Control RNAs were from U251 cells engineered with EGFR wild-type (w.t.) or EGFRvIII (vIII) genes. Data are representative of three independent PCR reactions. (D) Based on the amino acid sequence for anti-EGFRvIII mAbs, scFv genes were synthesized and fused to T-cell signaling domains from CD28 and CD3ζ followed by insertion into γ-retroviral vector MSGV1 (diagram). Retroviral vector preparations were produced and used to engineer primary human T cells as described in Materials and Methods. Transduced cells (UT, untransduced; 3C10 scFv, anti-EGFRvIII CAR vector based on murine mAb 3C10; 139 scFv, anti-EGFRvIII CAR vector based on human mAb 139) were subject to FACS analysis using reagents to detect the scFv (shaded histograms, unstained; middle histograms, isotype controls; right-shifted histograms, anti-CAR transduced cells). The percentage of CAR-positive cells was as shown. Histograms are representative of multiple (more than four) independent transductions. Similar results were obtained with anti-EGFRvIII CAR vector based on murine mAb L8A4 (data not shown). LTR, long terminal repeat; sd, splice donor; sa, splice acceptor; Ψ, extended packaging signal.