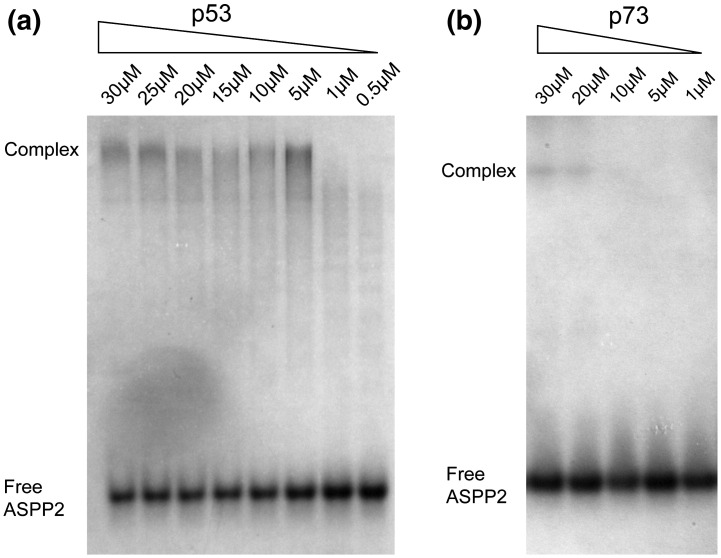
Fig. 5.
Native-gel mobility shift assay for ASPP2 binding. (a) Binding reactions contained 20 μM ASPP2 and decreasing concentrations of p53 DBD as indicated (p53 amino acids 94–312 were purified as described previously31). Binding was performed at 37 °C for 5 min followed by 30 min of incubation on ice. Proteins were buffered in 50 mM Tris, pH 7.2, 50 mM NaCl, and 5 mM DTT. Complexes were separated from unbound ASPP2 on a 10% polyacrylamide gel in Tris–glycine buffer at pH 8.3 and visualized with InstantBlue Coomassie stain. The p53 DBD has a net positive charge at this pH and does not enter the gel. (b) Under similar conditions, binding of the p73 DBD was not observed. A screen of different buffer conditions identified binding in 50 mM sodium phosphate, pH 7.2, 50 mM NaCl, 5 mM DTT, 50 mM l-arginine, and 50 mM l-glutamate. Binding reactions contained 20 μM ASPP2 and p73 amino acids 112–315 at decreasing concentrations as indicated. Binding was performed at 4 °C overnight and native PAGE was conducted as above.