Abstract
Background
Disadvantaged people include those experiencing economic, social or educational deprivation and, in some cases, those undergoing rapid transition from subsistence to industrial economies. Disadvantaged people worldwide are affected disproportionately by the global epidemic of diabetes. They are also at increased risk of kidney disease attributable to diabetes, and for many, the cost of managing their kidney disease far exceeds their available resources.
Methods
We review factors associated with disadvantage that may increase the risk of diabetic kidney disease, and the barriers to care that hinder attempts to provide an adequate therapeutic response.
Results and conclusions
A rapidly rising prevalence and magnitude of obesity among children and adults, increasing frequency of intrauterine exposure to diabetes, and inadequate access to healthcare are responsible, in part, for a surge in the frequency of diabetes and, in turn, diabetic kidney disease among disadvantaged people. These factors may also predispose to an earlier onset of diabetes and kidney disease, thereby perpetuating the disadvantage by reducing the earning potential of those affected through illness and disability.
Keywords: diabetic nephropathy, minorities, obesity, Type 2 diabetes in youth
Introduction
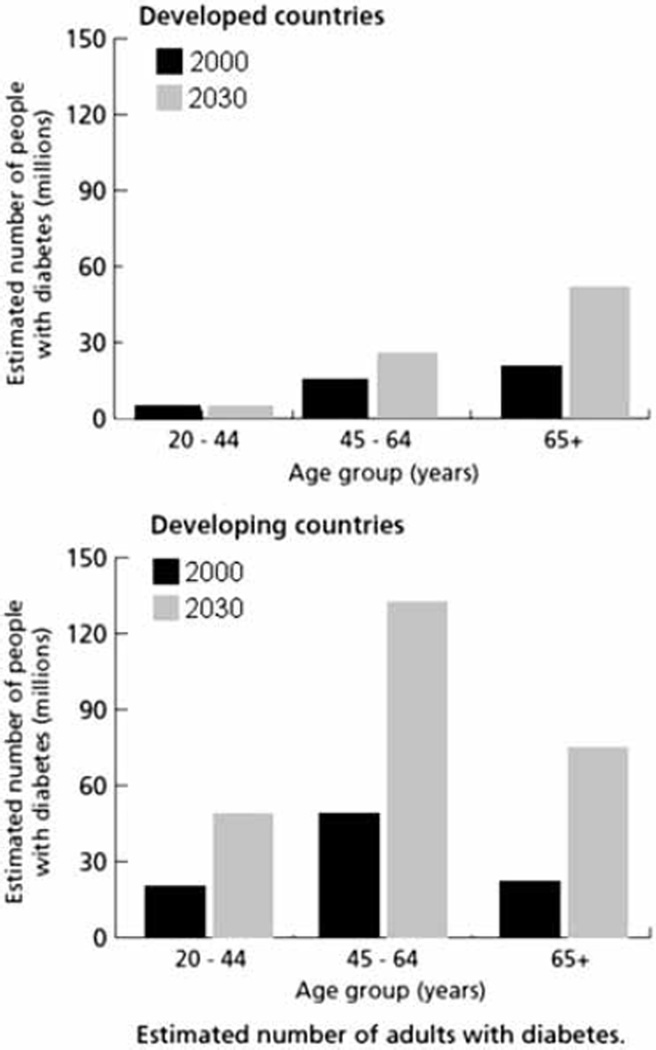
The World Health Organization (WHO) estimates that 180 million people worldwide have diabetes and projects the prevalence will double by the year 2030 [1]. Most of the increase will be due to Type 2 diabetes (T2DM). Most people with diabetes live in countries in the developing world that are also projected to have a much greater increase in the number of people with diabetes by 2030 than countries in the developed world (Figure 1). Many people in the developing world are undergoing rapid changes in their economies, lifestyles, and health, and many others experience similar changes as a result of immigration to the developed world. In both places, they are often disadvantaged with respect to their health, including kidney health. Factors contributing to the high rate of diabetic kidney disease in these people may include exposure to a broader range of environmental factors than people who lead more fortunate lives as well as differences in access to healthcare and genetic susceptibility to kidney disease.
Figure 1.
Estimated number of adults with diabetes in developed and developing countries. Adapted with permission of the World Health Organization [1].
This review is a condensed and updated version of previous reviews on the same topic [2, 3]. We examine risks for diabetic kidney disease of particular relevance and importance to disadvantaged people and the impact that limited resources may have on their ability to deal with the disease once it develops. We consider disadvantage to encompass people experiencing economic, social and educational deprivation, but also those undergoing rapid transition from subsistence to industrial economies, as such change is often disruptive and can lead to significant negative as well as positive consequences.
Environmental factors
Environmental factors that affect the development of diabetic kidney disease may be divided into those that influence risk before birth; those present after birth but before the development of diabetes; and those that add to the risk of progressive kidney disease in people with established diabetes. All of these factors are important in disadvantaged populations and are deeply intertwined with socioeconomic factors. Several of these factors that may be of greatest importance in disadvantaged populations are considered below.
Intrauterine exposures
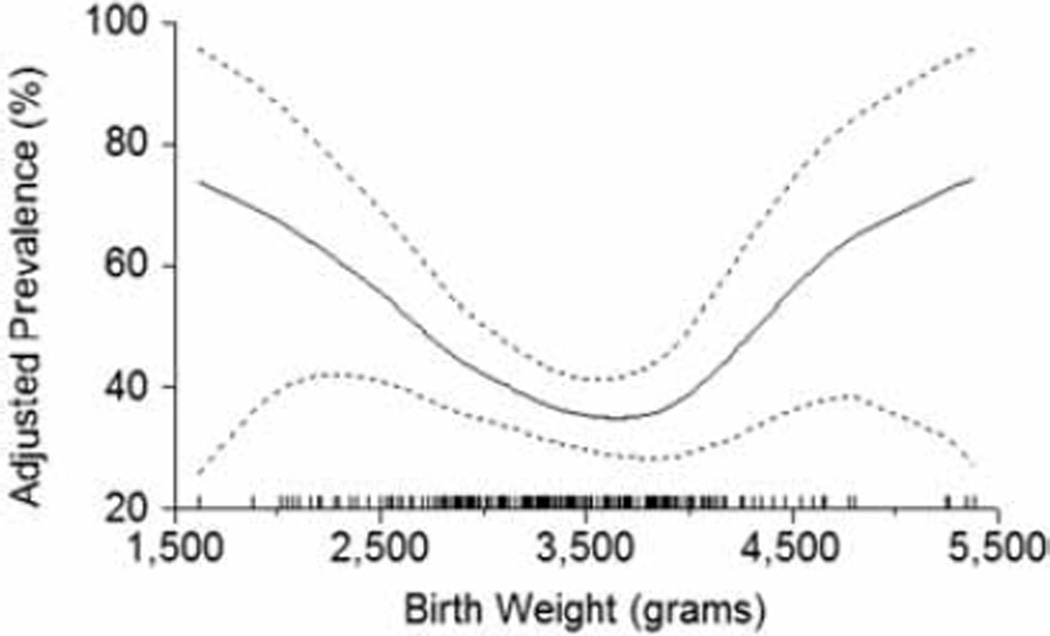
Maternal malnutrition is common among disadvantaged people and often causes intrauterine growth retardation in offspring, which may increase the risk of both T2DM [4, 5] and diabetic kidney disease [6, 7]. Intrauterine growth retardation affects a quarter of live births in developing countries [8] and represents a major potential cause of kidney disease in these populations. The mechanism for increased susceptibility to kidney disease among individuals with intrauterine growth retardation is thought to be impaired nephron development. With fewer nephrons and a correspondingly reduced filtration surface area, the resulting hyperfiltration may accelerate damage to the glomeruli initiated by diabetes [9]. In keeping with this hypothesis, elevated urinary albumin is more prevalent among diabetic Pima Indians with low birthweight [10] (Figure 2). Another manifestation of low nephron mass may be hypertension, which is found more often in persons of low birth weight, as reported in indigenous Australians [11].
Figure 2.
Prevalence of elevated urinary albumin excretion (albumin-to-creatinine ratio > 30 mg/g) in diabetic Pima Indians, by birth weight, adjusted for age, sex, duration of diabetes, HbA1C and mean arterial pressure. Dashed lines represent twice the point-wise asymptotic standard errors of the estimated curve, and the vertical tics on the x-axis are a frequency plot of birth weights. Values of the covariates were set to their sample means. Reprinted with permission from Oxford University Press [10].
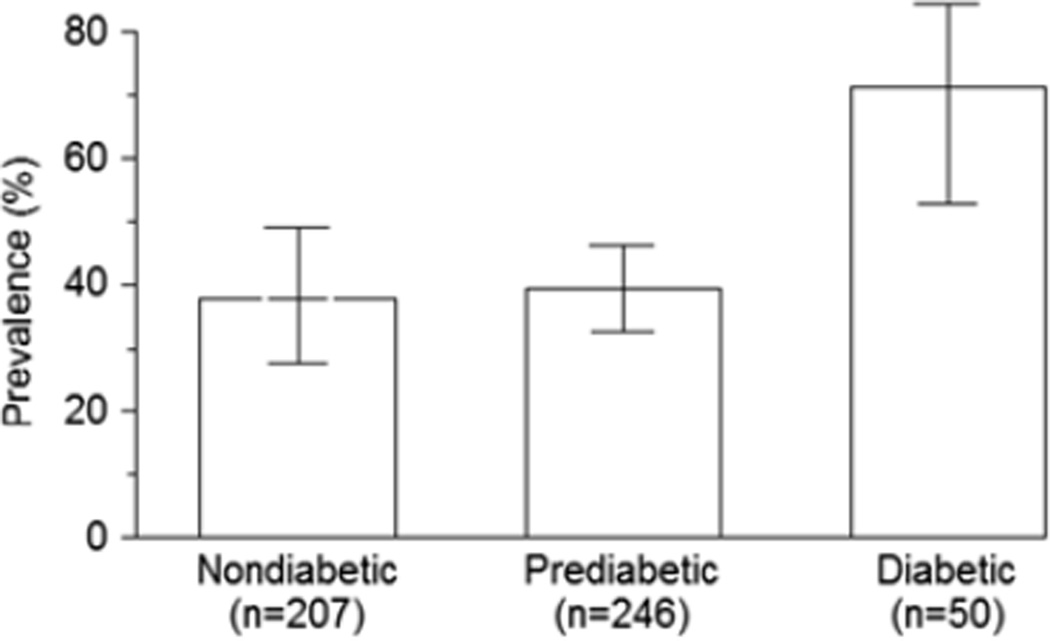
Exposure to maternal diabetes while in utero may also greatly increase the risk of diabetic nephropathy. In animal experiments, maternal diabetes during pregnancy reduces the number of nephrons in offspring [12]. In Pima Indians, the odds of elevated urinary albumin excretion were nearly four times as high in subjects exposed to diabetes in utero as in those exposed to a nondiabetic intrauterine environment (Figure 3) [13]. The largest increase in the number of people with diabetes during the childbearing years over the next several decades will occur in the developing world (Figure 1) [1], suggesting that the impact of intrauterine exposure to diabetes on the development of diabetic nephropathy will disproportionately affect disadvantaged people.
Figure 3.
Predicted prevalence (95% CIs) of elevated UAE (albumin-to-creatinine ratio > 30 mg/g) in diabetic Pima Indians, by maternal diabetes status, adjusted for age, sex, duration of diabetes, HbA1C and MAP. Values of the covariates were set to their sample means. In this figure, “Prediabetic” means that the mother did not have diabetes during pregnancy but developed Type 2 diabetes later in life. Copyright© 1998 American Diabetes Association. From Diabetes, Vol. 47, 1998; 1489–1494. Reprinted with permission from the American Diabetes Association [13].
Vitamin A (retinol) and its main derivative, retinoic acid, are involved in nephrogenesis, and rats exhibit a dose-dependent effect of Vitamin A on nephron number [14]. Both retinol and retinoic acid are potent stimulators of nephrogenesis, with a single injection of retinoic acid given to a pregnant rat at mid-gestation inducing supernumerary nephrons. Although human data on the relationship between vitamin A and nephron mass are not available, the existing animal data suggest that a mild vitamin A deficiency during pregnancy could lead to a nephron deficit in the offspring that enhances the risk of kidney disease. Vitamin A deficiency is not a major health problem in developed countries, but it is frequently encountered in developing countries, particularly in pregnant women, in whom intake of vitamin A may be inadequate to meet the increased demands encountered during pregnancy. Accordingly, fetal vitamin A deficiency may disproportionately affect disadvantaged populations and contribute to their increasing incidence rate of kidney disease.
Childhood obesity and youth-onset diabetes
The prevalence of overweight and obesity in both developed and developing countries has risen rapidly in recent years; 1.7 billion people worldwide are now classified as overweight or obese and at least 155 million of these are children, 22 million of whom are below 5 years of age [15]. The prevalence of overweight and obesity among children in developing countries adopting Western lifestyles has tripled in the past 20 years [16, 17]. Accompanying the increase in childhood obesity is an emerging epidemic of T2DM in youth [18], a problem presumably amplified in disadvantaged people by their higher frequency of intrauterine exposure to diabetes and its effect on development of diabetes and obesity in youth.
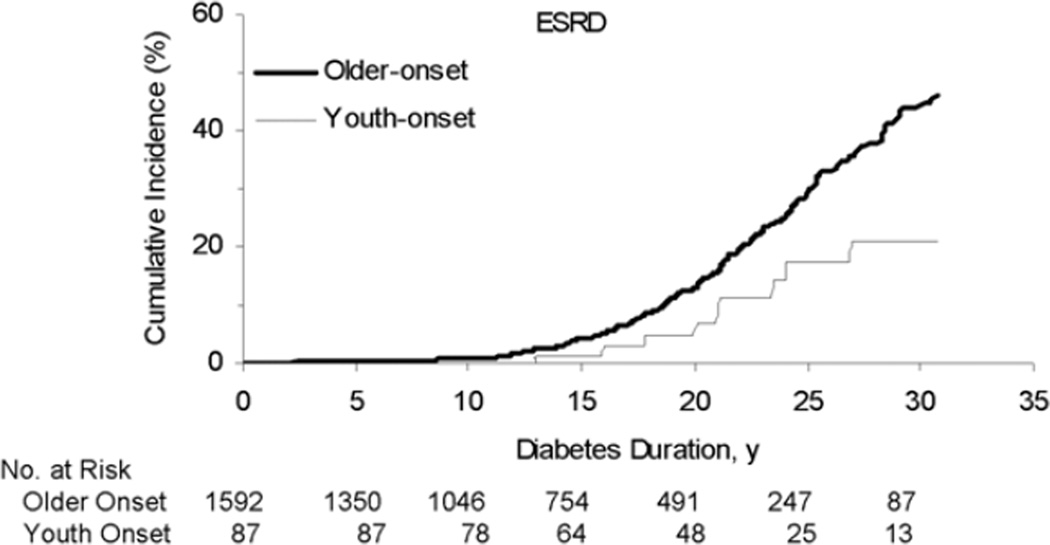
Individuals with youth-onset T2DM are at considerable risk for development of diabetic kidney disease [19, 20]. As in adults, the prevalence of renal complications of T2DM in youth correlates with duration of diabetes, so that the onset of diabetes in youth leads to the end-stage complications of diabetes, including kidney failure and death, much earlier in life (Figure 4) [21].
Figure 4.
Cumulative incidence of diabetic end-stage renal disease in Pima Indians by age at onset and duration of diabetes. Adapted with permission [21].
Infections
Once diabetes is established, the risk of diabetic kidney disease may be increased by chronic or repeated infections. A history of parasitic and bacterial infections, for example, was found more frequently among Australian Aboriginals who developed diabetic kidney disease [22]. The highest risk appears to result from repeated bacterial infections of the eyes, ears, nose, chest, skin and gut. Close living quarters and poor hygiene are largely responsible for the repeated bacterial and parasitic infections in the Aboriginal community.
Environmental toxins
Exposure to environmental toxins may not increase the risk of initiation of diabetic nephropathy, but may well accelerate its progression [23]. Disadvantaged populations may experience high levels of toxic exposures at work, since access to types of employment with less exposure may be limited by language and educational attainment. Those who live and work in rural environments may be exposed more frequently to nephrotoxins used in industrial agriculture including fertilizers, herbicides, and insecticides containing heavy metals such as arsenic. Urban workers may be exposed to hydrocarbons and heavy metals used in manufacturing, including cadmium, mercury, and lead.
Toxic exposures may also occur at home. As recent immigrants to cities, members of disadvantaged populations may be less likely to own land or have influence over land use. They are also more likely to live in neighborhoods where they are exposed to toxic chemicals at unhealthy levels. Cultural practices that arise in response to economic forces and social disruption may also lead to toxic exposures that harm the kidney. Moonshine, for example, is an alcoholic beverage made cheaply by distilling ethanol in radiators typically lined with lead and using copper pipe soldered with lead. Drinking moonshine distilled in such containers may result in lead nephropathy, which could also hasten progression of diabetic kidney disease [24].
Exposure to tobacco
In patients with T2DM, tobacco is an independent risk factor for onset and progression of diabetic nephropathy [25, 26], and smoking also increases the risk of diabetic end-stage renal disease (ESRD) [27]. Unfortunately, the prevalence of smoking is high in many developing countries [28]. Moreover, economic transition appears to increase the prevalence of cigarette smoking among Australian Aboriginals [29], and migration from rural to urban areas is associated with increased prevalence of tobacco abuse in China [30].
Socioeconomic factors
Economic transition can have different effects on socioeconomic status and on the risk of developing T2DM. People who successfully undergo economic transition – those who migrate to cities and take industrial jobs that pay well – experience an increase in socioeconomic status and greater access to food. In India, higher socioeconomic status increases the risk of diabetes [31], and in the US the risk of T2DM is highest among Hispanics of highest socioeconomic status [32]. On the other hand, higher socioeconomic status is generally associated with better education and with the resources to make healthier food choices. In the US, for example, the risk of T2DM among African-Americans is lowest in those of higher socioeconomic status [33]. Different levels of acculturation may, in part, explain the differential effects of socioeconomic status on the risk of T2DM in different populations [34].
Socioeconomic status also has an effect on the risk of diabetic complications. Once T2DM is diagnosed, diabetes duration, glycemic control, blood pressure, and microalbuminuria all predict future renal disease. Control of glycemia is dependent on a host of factors and poor glycemic control disproportionately affects many members of transitional and disadvantaged populations. In the Third National Health and Nutrition Examination Survey, glycemic control was poorer among Blacks and Mexican-Americans than in non-Hispanic whites [35]. In India, urban patients of lower socioeconomic status were more likely than those of higher status to develop complications of T2DM [36]. Poor glycemic control may also be exacerbated by a number of socioeconomic factors. Economic realities may make proper diet, diabetes testing equipment, and medicines unaffordable. Further, disadvantaged populations may have limited access to education, including health education. The benefits of medicines prescribed to control blood glucose and blood pressure may not be as clear to poorly educated patients, and the side effects of these medicines may discourage compliance, which has very real consequences in diabetic nephropathy. 36% of diabetic Mexican-American subjects ≥ 65 years old reported that they took their prescribed diabetes medicines inconsistently. Those who took their medicines inconsistently increased their odds of kidney disease by 1.6 compared to those with good consistency over a 7-year period, after controlling for age, sex, diabetes duration, education, income, marital status, language of interview, insurance status, medication type, cognitive function, presence of depressive symptoms, and activity of daily living [37].
Socioeconomic status correlates with progression of kidney disease in a number of disadvantaged populations. Among native Americans in Minnesota, family income and educational attainment are inversely associated with increased urine albumin excretion [38]. African-Americans with early decline in renal function are more likely to be of low socioeconomic status and have suboptimal health behaviors and suboptimal control of glucose and blood pressure [39]. Similarly, socioeconomic factors are strongly associated with the incidence of ESRD among indigenous Australians. Leaving school before the age of 15 years, unemployment, low household income, and over-crowding in the home were all found to correlate with increased risk of ESRD [40].
Barriers to care
There are many obstacles to the prevention, timely diagnosis, and treatment of T2DM and diabetic nephropathy in disadvantaged populations. Most important of these are the same lifestyle factors that increase the risk of T2DM and diabetic nephropathy. Changes in caloric balance that accompany the transition from a subsistence lifestyle (hunting, gathering or intensive agriculture) to an industrial lifestyle are not easily reversed once a diagnosis of T2DM is made. Hence, a dietary prescription may be difficult to implement and sustain for some members of transitional and disadvantaged populations [41]. The poverty that is associated with obesity and poor diet also limits the funds available to pay for healthy foods, exercise, access to a healthcare professional, and medicine once diabetes develops.
“Functional health literacy” is a measure of a patient’s ability to perform basic reading and numerical tasks required to function in the healthcare environment. It is distinct from educational level or language ability [42]. Poor functional health literacy limits effective communication between the patient and her/his healthcare provider, so patients are often confused about their disease and the process of care required to successfully manage their disease, and the practitioner is often frustrated by the patient’s lack of understanding and inability to implement treatment plans [43, 44].
Intertwined with impaired functional health literacy are complex patterns of health beliefs, motivations, and behaviors that also act as barriers to care of people with diabetes. Members of disadvantaged populations may often feel more comfortable using home remedies or seeking the help of traditional healers and, as a result, may seek the help of Western medicine only as a last resort. By the time such a patient comes to the attention of a practitioner of Western medicine, it is often too late to prevent nephropathy and the only therapy that remains is dialysis [45].
Limited access to healthcare presents a significant barrier to disadvantaged populations. For example, in small villages near Chennai, India, there are two physicians for every 25,000 people. While care and medicines are free, the patient must travel to the healthcare center and lose a day’s wages in order to receive care. As a result, few patients will seek the care of a physician unless they are in urgent need and they will not seek care for chronic diseases, such as T2DM, that may have no symptoms [46].
Even when barriers to care are removed, the risk of diabetic nephropathy may still remain elevated if standards of care are not met by the providers. Kaiser Permanente of Northern California provides unfettered access to providers and to pharmacy benefits for participants in its healthcare plan. Among 38,887 diabetic patients who were at high risk for diabetic nephropathy because of hypertension and/or elevated albuminuria, 61% received ACE inhibitors or ARBs. While there was no statistically significant difference in overall prescription rate for ACE inhibitors or ARBs among the racial and ethnic groups, African-Americans with albuminuria as the sole indication for therapy were less likely to have ACE inhibitors or ARBs prescribed [47]. Whether this treatment difference was due to a belief that ACE inhibitors or ARBs were less effective or contraindicated in African-Americans or to a greater unwillingness of African-American patients to receive these medicines is unknown. Among the Kaiser patients, Asians, African-Americans, and Hispanics had higher incidence rates of ESRD than non-Hispanic whites, despite equal access to care [48]. The persistence of ethnic disparities after removal of barriers to care suggests that other factors, including genetic, socioeconomic, environmental and attitudes or beliefs of providers or patients, may still have profound effects on outcomes of diabetes.
Conclusions
Diabetic kidney disease is a common and increasing problem worldwide, and it disproportionately affects disadvantaged people. Poverty and, paradoxically, the rapid emergence from poverty are, in part, responsible. Factors that contribute to the disproportionate burden include a higher frequency of damaging intrauterine exposures, including malnutrition and diabetes, a higher frequency of infectious diseases, and greater exposure to environmental toxins, including tobacco. These factors may also predispose to an earlier onset of diabetes and kidney disease, thereby perpetuating the disadvantage by reducing the earning potential of those affected through illness and disability. Unfortunately, particularly in developing countries, the cost of managing diabetic kidney failure already far exceeds the available resources. Accordingly, efforts to reduce the burden of diabetic kidney disease in disadvantaged populations should be directed towards diabetes prevention and early screening and intervention in those who already have diabetes.
Acknowledgment
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1. http://www.who.int/mediacentre/factsheets/fs312/en/
- 2.Nelson RG. Intrauterine determinants of diabetic kidney disease in disadvantaged populations. Kidney Int. 2003;83(Suppl):13–16. doi: 10.1046/j.1523-1755.63.s83.4.x. [DOI] [PubMed] [Google Scholar]
- 3.Weil EJ, Nelson RG. Diabetic Kidney Disease in Transitional and Disadvantaged Populations. In: Cortes P, Mogensen CE, editors. The Diabetic Kidney. Totowa, New Jersey: Humana Press; 2006. [Google Scholar]
- 4.Phipps K, Barker DJ, Hales CN, Fall CH, Osmond C, Clark PM. Fetal growth and impaired glucose tolerance in men and women. Diabetologia. 1993;36:225–228. doi: 10.1007/BF00399954. [DOI] [PubMed] [Google Scholar]
- 5.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 7.Lopes AA, Port FK. Differences in the patterns of age-specific black/white comparisons between end-stage renal disease attributed and not attributed to diabetes. Am J Kidney Dis. 1995;25:714–721. doi: 10.1016/0272-6386(95)90547-2. [DOI] [PubMed] [Google Scholar]
- 8.de Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52(Suppl):5–15. [PubMed] [Google Scholar]
- 9.Lemley KV. A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int. 2003;83(Suppl):38–42. doi: 10.1046/j.1523-1755.63.s83.9.x. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RG, Morgenstern H, Bennett PH. Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. Am J Epidemiol. 1998;148:650–656. doi: 10.1093/aje/148.7.650. [DOI] [PubMed] [Google Scholar]
- 11.Singh GR, Hoy WE. Kidney volume, blood pressure, and albuminuria: findings in an Australian aboriginal community. Am J Kidney Dis. 2004;43:254–259. doi: 10.1053/j.ajkd.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes. 1999;48:2240–2245. doi: 10.2337/diabetes.48.11.2240. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RG, Morgenstern H, Bennett PH. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes. 1998;47:1489–1493. doi: 10.2337/diabetes.47.9.1489. [DOI] [PubMed] [Google Scholar]
- 14.Merlet-Benichou C. Influence of fetal environment on kidney development. Int J Dev Biol. 1999;43:453–456. [PubMed] [Google Scholar]
- 15. http://www.iotf.org/childhoodobesity.asp.
- 16.Poskitt EM. Countries in transition: underweight to obesity non-stop? Ann Trop Paediatr. 2009;29:1–11. doi: 10.1179/146532809X401971. [DOI] [PubMed] [Google Scholar]
- 17.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 19.Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care. 2003;26:76–81. doi: 10.2337/diacare.26.1.76. [DOI] [PubMed] [Google Scholar]
- 20.Maahs DM, Snively BM, Bell RA, Dolan L, Hirsch I, Imperatore G, Linder B, Marcovina SM, Mayer-Davis EJ, Pettitt DJ, Rodriguez BL, Dabelea D. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2007;30:2593–2598. doi: 10.2337/dc07-0450. [DOI] [PubMed] [Google Scholar]
- 21.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 22.Thomas M. Antecedents of renal disease in Aborigines. Nephrology (Carlton) 2004;9:113–116. doi: 10.1111/j.1440-1797.2004.00344.x. [DOI] [PubMed] [Google Scholar]
- 23.Yaqoob M, Patrick AW, McClelland P, Stevenson A, Mason H, Percy DF, White MC, Bell GM. Occupational hydrocarbon exposure and diabetic nephropathy. Diabet Med. 1994;11:789–793. doi: 10.1111/j.1464-5491.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsaih SW, Korrick S, Schwartz J, Amarasiriwardena C, Aro A, Sparrow D, Hu H. Lead, diabetes, hypertension, and renal function: the normative aging study. Environ Health Perspect. 2004;112:1178–1182. doi: 10.1289/ehp.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biesenbach G, Grafinger P, Janko O, Zazgornik J. Influence of cigarette-smoking on the progression of clinical diabetic nephropathy in type 2 diabetic patients. Clin Nephrol. 1997;48:146–150. [PubMed] [Google Scholar]
- 26.Orth SR, Stockmann A, Conradt C, Ritz E, Ferro M, Kreusser W, Piccoli G, Rambausek M, Roccatello D, Schafer K, Sieberth HG, Wanner C, Watschinger B, Zucchelli P. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int. 1998;54:926–931. doi: 10.1046/j.1523-1755.1998.00067.x. [DOI] [PubMed] [Google Scholar]
- 27.Muhlhauser I. Cigarette smoking and diabetes: an update. Diabet Med. 1994;11:336–343. doi: 10.1111/j.1464-5491.1994.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 28.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 29.Perkins JJ, Sanson-Fisher RW, Blunden S, Lunnay D, Redman S, Hensley MJ. The prevalence of drug use in urban aboriginal communities. Addiction. 1994;89:1319–1331. doi: 10.1111/j.1360-0443.1994.tb03311.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Li X, Stanton B, Fang X, Lin D, Cole M, Liu H, Yang H. Cigarette smoking among rural-to-urban migrants in Beijing, China. Prev Med. 2004;39:666–673. doi: 10.1016/j.ypmed.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran A, Snehalatha C, Vinitha R, Thayyil M, Kumar CK, Sheeba L, Joseph S, Vijay V. Prevalence of overweight in urban Indian adolescent school children. Diabetes Res Clin Pract. 2002;57:185–190. doi: 10.1016/s0168-8227(02)00056-6. [DOI] [PubMed] [Google Scholar]
- 32.Pazmino PA, Pazmino AK. Diabetic renal failure in Texas: influence of ethnicity and household income. Tex Med. 2003;99:57–65. [PubMed] [Google Scholar]
- 33.Brancati FL, Whelton PK, Kuller LH, Klag MJ. Diabetes mellitus, race, and socioeconomic status. A population-based study. Ann Epidemiol. 1996;6:67–73. doi: 10.1016/1047-2797(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 34.Hazuda HP, Haffner SM, Stern MP, Eifler CW. Effects of acculturation and socioeconomic status on obesity and diabetes in Mexican Americans. The San Antonio Heart Study. Am J Epidemiol. 1988;128:1289–1301. doi: 10.1093/oxfordjournals.aje.a115082. [DOI] [PubMed] [Google Scholar]
- 35.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. The Third National Health and Nutrition Examination Survey 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran A, Snehalatha C, Vijay V, King H. Impact of poverty on the prevalence of diabetes and its complications in urban southern India. Diabet Med. 2002;19:130–135. doi: 10.1046/j.1464-5491.2002.00656.x. [DOI] [PubMed] [Google Scholar]
- 37.Kuo YF, Raji MA, Markides KS, Ray LA, Espino DV, Goodwin JS. Inconsistent use of diabetes medications, diabetes complications, and mortality in older Mexican Americans over a 7-year period: data from the Hispanic established population for the epidemiologic study of the elderly. Diabetes Care. 2003;26:3054–3060. doi: 10.2337/diacare.26.11.3054. [DOI] [PubMed] [Google Scholar]
- 38.Kasiske BL, Rith-Najarian S, Casper ML, Croft JB. American Indian heritage and risk factors for renal injury. Kidney Int. 1998;54:1305–1310. doi: 10.1046/j.1523-1755.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 39.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159:1777–1783. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 40.Cass A, Cunningham J, Snelling P, Wang Z, Hoy W. End-stage renal disease in indigenous Australians: a disease of disadvantage. Ethn Dis. 2002;12:373–378. [PubMed] [Google Scholar]
- 41.Shobhana R, Begum R, Snehalatha C, Vijay V, Ramachandran A. Patients’ adherence to diabetes treatment. J Assoc Physicians India. 1999;47:1173–1175. [PubMed] [Google Scholar]
- 42.Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. JAMA. 1999;281:552–557. [PubMed] [Google Scholar]
- 43.Georges CA, Bolton LB, Bennett C. Functional health literacy: an issue in African-American and other ethnic and racial communities. J Natl Black Nurses Assoc. 2004;15:1–4. [PubMed] [Google Scholar]
- 44.Schillinger D, Bindman A, Wang F, Stewart A, Piette J. Functional health literacy and the quality of physician-patient communication among diabetes patients. Patient Educ Couns. 2004;52:315–323. doi: 10.1016/S0738-3991(03)00107-1. [DOI] [PubMed] [Google Scholar]
- 45.Larkey LK, Hecht ML, Miller K, Alatorre C. Hispanic cultural norms for health-seeking behaviors in the face of symptoms. Health Educ Behav. 2001;28:65–80. doi: 10.1177/109019810102800107. [DOI] [PubMed] [Google Scholar]
- 46.Mani MK. Experience with a program for prevention of chronic renal failure in India. Kidney Int. 2005;94(Suppl):75–78. doi: 10.1111/j.1523-1755.2005.09419.x. [DOI] [PubMed] [Google Scholar]
- 47.Rosen AB, Karter AJ, Liu JY, Selby JV, Schneider EC. Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high-risk clinical and ethnic groups with diabetes. J Gen Intern Med. 2004;19:669–675. doi: 10.1111/j.1525-1497.2004.30264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. Erratum in: JAMA 2002 288 46. [DOI] [PubMed] [Google Scholar]