Abstract
Medial amygdaloid tissue, taken from female rats immediately after birth, was transplanted into the anterior chamber of the eye in adult ovariectomized host rats in order to elucidate the influence of estrogen on synapse formation without contribution of neural afferents. After injections of estradiol benzoate or oil vehicle to the hosts for 20 successive days, the grafts were processed for semiquantitative electron microscopic study to examine synaptic density in the neuropil. The number of synapses on dendritic shafts vs. dendritic spines was not significantly different in the control group. In contrast, in the grafts exposed to estrogen, shaft synapses occurred more frequently than spine synapses. Synaptic density on shafts was significantly greater in these grafts than that in the controls, although the density on spines did not differ between the two groups. These data show that estrogen affects the medial amygdaloid neurons themselves and specifically facilitates the formation of dendritic shaft synapses in oculo. Our previous report raises the possibility that the specific increase of shaft synapses induced by sex steroids is involved in the process of sexual differentiation of neuronal networks from the inherently feminine pattern to the masculine pattern in the medial amygdala. Therefore, the present findings may provide evidence that sexual differentiation triggered by sex steroids is accomplished by intrinsic factors in the neurons of the medial amygdala.
Full text
PDF
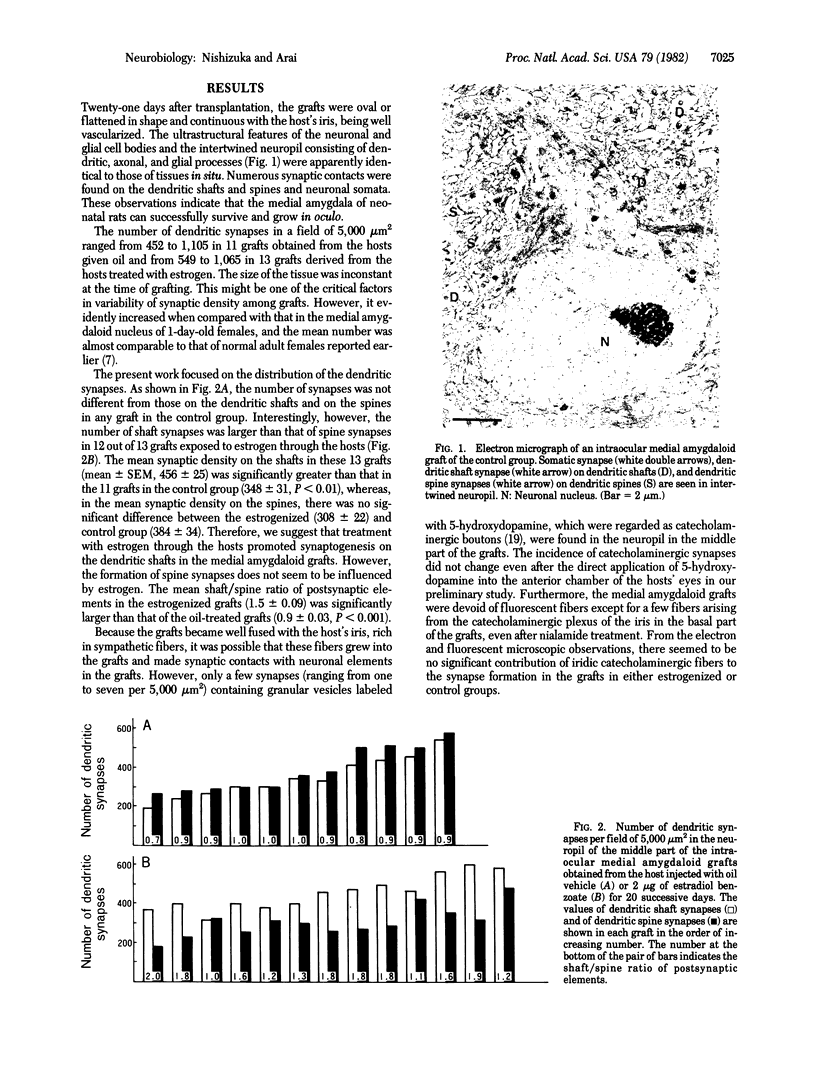

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimatsu Y., Seto A., Amano T. Sexual dimorphism in alpha-bungarotoxin binding capacity in the mouse amygdala. Brain Res. 1981 Jun 1;213(2):432–437. doi: 10.1016/0006-8993(81)90249-3. [DOI] [PubMed] [Google Scholar]
- Breedlove S. M., Arnold A. P. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980 Oct 31;210(4469):564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Koziell D. A., Moore R. Y. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978 Aug 1;180(3):509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Hamberger B., Hökfelt T. Distribution of noradrenaline nerve terminals in cortical areas of the rat. Brain Res. 1968 Apr;8(1):125–131. doi: 10.1016/0006-8993(68)90175-3. [DOI] [PubMed] [Google Scholar]
- Gorski R. A., Gordon J. H., Shryne J. E., Southam A. M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978 Jun 16;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Jaeger C. B., Lund R. D. Transplantation of embryonic occipital cortex to the brain of newborn rats. An autoradiographic study of transplant histogenesis. Exp Brain Res. 1980;40(3):265–272. doi: 10.1007/BF00237791. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978 Mar 15;178(2):255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Krieger M. S., Conrad L. C., Pfaff D. W. An autoradiographic study of the efferent connections of the ventromedial nucleus of the hypothalamus. J Comp Neurol. 1979 Feb 15;183(4):785–815. doi: 10.1002/cne.901830408. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Naftolin F. Sexual differentiation of the central nervous system. Science. 1981 Mar 20;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Arai Y. Effect of androgen on sexual differentiation of synaptic organization in the hypothalamic arcuate nucleus: an ontogenetic study. Neuroendocrinology. 1981 Sep;33(3):166–169. doi: 10.1159/000123223. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Arai Y. Sexual dimorphism in 'wiring pattern' in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Res. 1980 May 19;190(1):238–242. doi: 10.1016/0006-8993(80)91173-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka M., Arai Y. Organizational action of estrogen on synaptic pattern in the amygdala: implications for sexual differentiation of the brain. Brain Res. 1981 Jun 1;213(2):422–426. doi: 10.1016/0006-8993(81)90247-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka M., Arai Y. Sexual dimorphism in synaptic organization in the amygdala and its dependence on neonatal hormone environment. Brain Res. 1981 May 11;212(1):31–38. doi: 10.1016/0006-8993(81)90029-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka M. Topography of the neurons responding to estrogen in the hypothalamic arcuate nucleus of immature female mice. Brain Res. 1978 Aug 18;152(1):31–40. doi: 10.1016/0006-8993(78)90132-4. [DOI] [PubMed] [Google Scholar]
- Olson L., Malmfors T. Growth characteristics of adrenergic nerves in the adult rat. Fluorescence histochemical and 3H-noradrenaline uptake studies using tissue transplantations to the anterior chamber of the eye. Acta Physiol Scand Suppl. 1970;348:1–112. [PubMed] [Google Scholar]
- Pfaff D., Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973 Sep 15;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Raisman G., Field P. M. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973 May 17;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Hopkins D. A. Amygdala afferents from the mediobasal hypothalamus: an electrophysiological and neuroanatomical study in the rat. Brain Res. 1977 Feb;121(2):201–213. doi: 10.1016/0006-8993(77)90147-0. [DOI] [PubMed] [Google Scholar]
- Scalia F., Winans S. S. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975 May 1;161(1):31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Seiger A., Olson L. Brain tissue transplanted to the anterior chamber of the eye: 3. Substitution of lacking central noradrenaline input by host iris sympathetic fibers in the isolated cerebral cortex developed in oculo. Cell Tissue Res. 1975 Jun 13;159(3):325–338. doi: 10.1007/BF00221780. [DOI] [PubMed] [Google Scholar]
- Stenevi U., Björklund A., Kromer L. F., Paden C. M., Gerlach J. L., McEwen B. S., Silverman A. J. Differentiation of embryonic hypothalamic transplants cultured on the choroidal pia in brains of adult rats. Cell Tissue Res. 1980;205(2):217–228. doi: 10.1007/BF00234681. [DOI] [PubMed] [Google Scholar]
- Swanson L. W. An autoradiographic study of the efferent connections of the preoptic region in the rat. J Comp Neurol. 1976 May 15;167(2):227–256. doi: 10.1002/cne.901670207. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D., Gerlach J. L., McEwen B. S. Autoradiographic localization of [3H]estradiol related to steroid responsiveness in cultures of the newborn mouse hypothalamus and preoptic area. Brain Res. 1980 Feb 24;184(2):517–522. doi: 10.1016/0006-8993(80)90820-3. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Thoenen H. Electronmicroscopic localization of 5-hydroxydopamine (3,4,5-trihydroxy-phenyl-ethylamine), a new 'false' sympathetic transmitter. Experientia. 1967 Sep 15;23(9):743–745. doi: 10.1007/BF02154151. [DOI] [PubMed] [Google Scholar]
- Woodward D. J., Seiger A., Olson L., Hoffer B. J. Intrinsic and extrinsic determinants of dendritic development as revealed by Golgi studies of cerebellar and hippocampal transplants in oculo. Exp Neurol. 1977 Dec;57(3):984–998. doi: 10.1016/0014-4886(77)90122-4. [DOI] [PubMed] [Google Scholar]