Abstract
Targeted genomic manipulation using embryonic stem (ES) cells has not yet been achieved in zebrafish, although methods for zebrafish ES cell culture has been described in literature. The knowledge of pluripotency markers in this species is almost nonexistent and this is a very limiting factor in the definition of the ideal culture conditions for ES cells. Here, we studied the expression of several genes associated with pluripotency in zebrafish embryonic cells versus differentiated cells and the expression of some of these genes is recorded throughout embryonic development. Some of the commonly accepted pluripotency markers are also tested in embryonic cells, transient embryonic cell cultures, and differentiated cells. Our results support the hypothesis that stage-specific embryonic antigen 1 (SSEA1) is a marker that precedes the expression of pluripotency genes in a zebrafish embryonic cell colony, in the same way that SOX2 precedes nestin expression in those colonies that have already started differentiation toward neurons. We consider this study a step forward in the knowledge of zebrafish pluripotency markers and, therefore, an important tool for the monitoring of zebrafish embryonic cell cultures.
Introduction
In recent years, zebrafish have become an important model system for science. New mutant and transgenic lines are constantly emerging and publications using this biological model are increasing exponentially. In this scenario, zebrafish may possibly be on the same level as mice in the near future. However, there are still some deficiencies that need to be overcome, particularly the establishment of real embryonic stem (ES) cell cultures that will enable knockout and knockin technologies to be used in this species. Zinc finger nucleases have been successfully used for targeting gene inactivation in zebrafish,1,2 and targeted insertions are being explored using this technology. However, despite all the efforts made to establish zebrafish ES cell cultures,3 cell-mediated gene targeting has not been achieved. It has been published that some of these cultures remain pluripotent and germline competent for some passages4 and homologous recombination has also been carried out, demonstrating the potential of using these cultures in a cell-mediated gene targeting approach.5 However, despite these great advances, only zebrafish blastula cell lines have been established rather than ES cell lines.6 One of the main issues at the root of these unsuccessful trials is the lack of knowledge of zebrafish pluripotency markers. It is common practice to assume that markers associated with pluripotency in other species can be directly extrapolated to zebrafish. However, it is well known that this is not necessarily true. As an example, stage-specific embryonic antigen 1 (SSEA1) is a pluripotency marker in mouse cells, but in humans, it is a differentiation marker.7,8 There are some recent studies that address pluripotency similarities and transcriptional networks in zebrafish blastomeres and mouse ES cells. Onichtchouk et al.9 studied the similarity of Pou5f1 trasncriptional targets in zebrafish and mice, and Okuda et al.10 also reported that B1 sox functions are central to coordinate diverse embryonic processes. However, nothing is known about the behavior of the pluripotency markers in the transient zebrafish embryonic cultures. More detailed knowledge of the specific markers that can be used to determine pluripotency is crucial to successfully establish ES cell cultures in this species.
Here, we describe the expression of several pluripotency-associated genes in zebrafish embryonic cells (at the developmental stage at which transient embryonic cell cultures are derived5) versus differentiated cells. Also, the expression of some of these genes is recorded throughout embryonic development, and some common pluripotency markers are also tested in embryonic cells, differentiated cells, and embryonic cells that have been cultured using LDF media (50% Libowitz's L15, 35% Dulbecco's Modified Eagle's, 15% Ham's F12) supplemented with B27 and N2 serum-free supplements with two inhibitors GHIR99021 and PD184352. Immunohistochemistry has been used to check for the presence of the protein in the embryo and cell cultures. We have observed that pou5f1 (the homolog of mammalian oct4) showed 14-fold higher expression in blastomeres than in adult somatic cells and seems to have an important role in pluripotency; however, the protein OCT4 has been detected in the embryo, but it was not detected in the cultures. On the other hand, sox2 plays an important role in neuronal differentiation, but not in pluripotency. We consider this study a basic pillar for the future establishment of stable zebrafish ES cell cultures.
Materials and Methods
Zebrafish maintenance
Zebrafish (Danio rerio), AB strain (one of the common wild-type strains in zebrafish), were maintained in 10-L aquaria with a recirculating water system (Aquatic Habitats) under standard conditions. The fish were fed twice daily with dry food and live artemia.
Embryo collection and transient embryonic cell culture
For embryo collection, males and females were transferred to a 1.5-L breeding tank (Aquatic Habitats) with a ratio of one male to two females and kept separated until the next morning when the barrier was removed. Once the embryos were collected, in the cleavage period, they were washed for 2 min with a 0.5% solution of bleach, rinsed twice with embryo medium (EM; 7.5 mM NaCl, 0.25 mM KCl, 0.5 mM MgSO4, 0.7 mM KH2PO4, 0.02 mM Na2HPO4, 0.5 mM CaCl2, and 0.35 mM NaHCO3), and kept in fresh EM at 28°C until they reached the appropriate stage for the experiments (oblong stage [3 h 40 min]11 for cell culture, and 16-cell, 256-cell, oblong, 50% epiboly, or 24 h postfertilization stage for gene expression).
For blastoderm recovery, embryos were dechorionized using 2 mg/mL pronase E (Sigma) in EM. After 5 min, they were gently swirled in a Petri dish to separate them from the chorion and then were placed in fresh EM. Blastoderms were carefully separated from the yolk with sterile fine forceps and were then placed in sterile L15 medium until plated.
The blastoderms were plated over Matrigel (to retard differentiation) or laminin (to induce neuronal differentiation)-coated dishes in LDF media supplemented (1:300, v/v) with B27 and N2 serum-free supplements (Invitrogen) with two inhibitors, glycogen synthase kinase 3 (GSK3) (CHIR99021) and MEK-1 (PD184352) (kindly provided by A. Smith), at concentrations 3 and 0.5 μM, respectively.
RNA extraction and cDNA synthesis
Total RNA was extracted from zebrafish embryos using TRIZOL® according to the manufacturer's guidelines. RNA concentration was measured using a NanoDrop spectrophotometer (ND-1000; Thermo Scientific) and 0.2 μg total RNA was used for reverse transcription in a 20 μL mixture containing 1 μL of 50 μM Oligo(dT) primer, 2 μL of 10 mM dNTP Mix, 4 μL of 5 × cDNA synthesis buffer, 1 μL of 0.1 M DTT, 1 μL RNAseOUT, 1 μL cloned AMV RT (15 units/μL) (Invitrogen), and diethylene pyrocarbonate (DEPC)-treated water to 20 μL. The reverse transcription was conducted at 50°C for 50 min and 85°C for 5 min, and the samples were stored at −20°C until use. A pool of 100 embryos was used for each replicate. Four replicates were made.
Real-time quantitative polymerase chain reaction
Polymerase chain reaction (PCR) products were detected by measuring the increase in fluorescence caused by the binding of SYBR GREEN dye to dsDNA in the reaction tube. Ten microliters of SYBR® GreenER™ qPCR SuperMix (Invitrogen; 11760-500) containing hot-start Taq DNA polymerase, SYBR® GreenER™ fluorescent dye, 1 μM ROX Reference Dye, MgCl2, dNTPs (with dUTP instead of dTTP), UDG, and stabilizers were added to 6 μL water, 2 μL sample, and 1 μL of each primer (10 μM). The primers were designed using the Primer Express Software from Applied Biosystems. Primer pairs were chosen to minimize dimerization and were tested prior to the experiments. Relative expression of the PCR products was determined using the ΔΔCt method12 using rpl13A as housekeeping gene. Each sample was run in triplicate and the mean Ct was used in the equation. The primer sets used are shown in Table 1.
Table 1.
Forward and Reverse Primers Used in the Quantification of Relative Expression by Quantitative Polymerase Chain Reaction of Zebrafish Genes
| Gene | Accession no. | Primer F | Primer R |
|---|---|---|---|
| pou5f1 | NM1311121 | GGTTCGGAAGCCCAGGATT | TGAGCTGAGGGAATGTTTTGC |
| sox2 | NM213118,1 | ACCCCGGAGGAAAACCAA | CCCGGCAGGGTGTACTTG |
| zic3 | NM001001950,2 | CCCTGGGCTGGGACTCA | CTTGAAGGCAGCCGAGTGA |
| klf4 | NM131723 | GAACCACTGCGGGCAAAT | GATGGTGGAGTCAGCATCACA |
| c-myc | L11710 | CGTCAACGCGGCATGA | GATTGTTGCTAGCCTCAAGTCGTA |
| sall4 | NM001080609 | CTCCCAGAGACCTTCTTCATCAG | GACCGAACATGCCAGAAGAAA |
| tert | NM001083866 | CGACAGCAAACCGAAAAAACTT | CGACTGAATAGCGGCACCAT |
| hsp60 | NM_181330 | GGTGAGGACGGCACTGCTA | TTCAGCGGTGGACAAGAGAGA |
| hsp90a1 | NM_131328 | TGAACTGATCCCAGACCAGAAA | CAATGCCGGTGTCGATGAT |
Gene names and accession numbers are specified.
Flow cytometry analysis
Cells were incubated for 30 min with 1:100 anti-SSEA1 mouse IgM antibody (Iowa) followed by 30 min of incubation with a second antibody, 1:100 goat anti-mouse-FITC. The cells were then washed twice in PBS/0.5% BSA/5 mM EDTA. As negative control, cells were incubated only with the secondary antibody. Immediately after, samples were processed in a Moflo cell sorter (DakoCytomation) and adjusted for the detection of FITC fluorescence (Ex 494 nm/Em 518 nm). All analyses were performed by applying Summit software.
Alkaline phosphatase assay
Alkaline phosphatase activity was detected in embryos using an Alkaline Phosphatase Detection Kit (Chemicon) according to the manufacturer's instructions.
Immunohistochemistry
For immunohistochemistry analysis, samples were fixed in 4% paraformaldehyde (PFA) at 4°C overnight (for zebrafish embryos) or 1 h (for cells) and then washed in Tris-buffered saline (TBS). After fixation, they were mechanically dechorionized, and antigen retrieval with citrate buffer was done at 60°C (pH 8.5) for 30 min in the embryo samples only. Then, the samples were washed in TBS and incubated in blocking buffer (TBS with 0.5% triton and 6% donkey serum) for 30 min at room temperature and washed three times in TBS. The samples were exposed to primary antibodies: SSEA1 mouse IgM antibody (Iowa MC-480), alpha tubulin mouse IgG antibody (Sigma T6074), phospho histone H3 rat IgG antibody (Sigma H9908), OCT4 goat IgG (Santa Cruz sc8628), cytoqueratin rabbit IgG (Dako Z0662), SOX2 (Chemicon AB5603), and nestin rabbit IgG (Chemicon AB5922) in TBS with 6% serum for 24 h at 4°C in a humid chamber and then washed three times in TBS followed by incubation with the secondary antibody at 37°C in a humid chamber (2 h for embryos and 1 h for cells) as well as an anti-goat IgG-FITC (Jackson) for the embryos. Finally, the samples were washed in TBS three times, and incubation with anti-FITC-Alexa488 goat IgG-Alexa488 (Invitrogen) for 1 h at 37°C was done with zebrafish embryos. After the washing steps, the samples were exposed to DAPI 1:10,000 for 15 min. Immunostained samples were examined by confocal microscopy (Leica SP5 AOBS).
Statistical analysis
Results were expressed as mean ± SD. To evaluate the statistically significant differences between the groups (p < 0.05), a one-way analysis of variance was used, followed by a post hoc Student-Newman-Keuls (SNK) test. Student's t-test was used to compare the expression of adult somatic cells with the expression of blastomeres for each marker.
Results
Relative gene expression of pluripotency-associated markers in zebrafish blastomeres and throughout embryo development
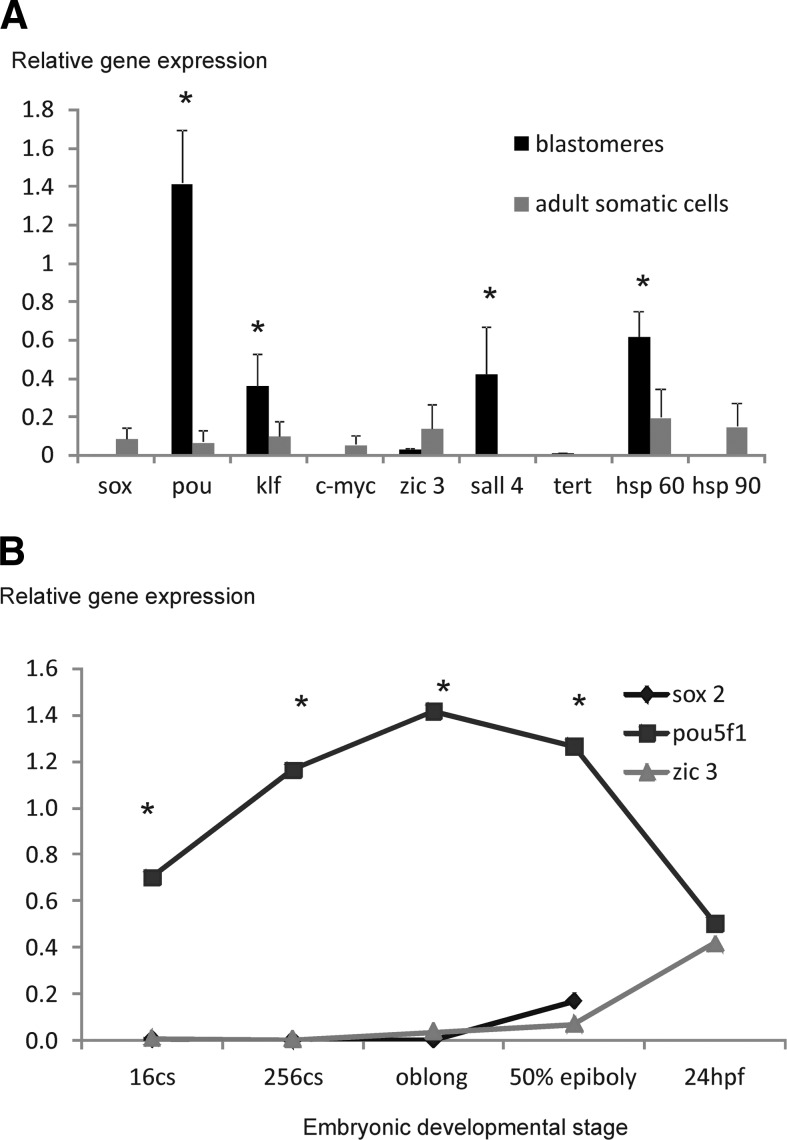
To determine the possible candidate genes to be considered pluripotency markers in zebrafish, we analyzed the relative expression of a set of genes, commonly used to characterize mouse and human ES cells, in zebrafish oblong-staged embryo blastomeres. This developmental stage has been considered the optimal range to derive ES cell–like cultures.5 The panel of genes that has been chosen includes the four reprogramming factors oct4 (POU class 5 homoeobox1, also called pou5f1), sox2 (sex-determining region Y box 2), klf4 (Kruppel-like factor 4), and c-myc (proto-oncogene myc) as well as zic3, sal-like 4 (sall4), the homolog of telomerase reverse transcriptase, tert, and two heat shock proteins hsp60 and hsp90a1, which are associated with pluripotency (Table 1). Four of these genes, pou5f1, klf4, sall4, and hsp60, showed higher expression (ranging from 4-fold for klf4 to 14-fold for pou5f1) in blastomeres than in adult differentiated somatic cells (Fig. 1A). Intriguingly, some others such as sox2 and zic3 showed the opposite tendency. When the expression of these genes (pou5f1, sox2, and zic3) was analyzed throughout development, it was clearly seen that pou5f1 expression was higher than sox2 and zic3 expression in all the developmental stages analyzed (16-cell embryo, 256-cell embryo, oblong stage, 50% epiboly, and 24 h postfertilization), reaching a peak in the oblong stage (Figs. 1B and 2). However, the sox2 and zic3 relative expression levels in the oblong stage are minimal, undergoing a slight increase in 50% epiboly embryos (Fig. 1B). On the other hand, zic3 reached similar levels of expression to pou5f1 at 24 h postfertilization (Fig. 1B).
FIG. 1.
(A) Relative gene expression for pluripotency-associated markers in zebrafish blastomeres from oblong-staged embryos (black) and adult somatic cells (gray). Rpl13 was the reference gene. Results are expressed as mean ± SD (n = 3). Significant upregulation of pluripotency markers in embryonic cells are shown with asterisks. (B) The relative gene expression for the pluripotency-associated markers sox2 (rhombus), pou5f1 (squares), and zic3 (triangles) throughout embryonic development. Significant differences in gene expression in the different developmental stages are shown with asterisks.
FIG. 2.
POU5 in zebrafish oblong-staged embryos (POU5, green; DAPI, blue; cytokeratin, red; phosphohistone H3, yellow). Color images available online at www.liebertonline.com/zeb
Expression pattern of pluripotency-associated markers in zebrafish embryos and transient embryonic cell cultures
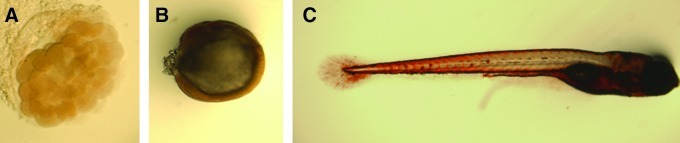
We have tested the validity of some of the markers associated with mammalian ES cells and employed them in the characterization of zebrafish embryonic cells. Zebrafish embryos were evaluated for the presence of alkaline phosphatase activity, SSEA1, as well as the homolog of the mammalian oct 4 (pou5f1). Our results demonstrated that differentiated embryonic cells that do not retain pluripotency ability are clearly positive for alkaline phosphatase activity (Fig. 3), and therefore, this assay is not suitable for pluripotency evaluation purposes.
FIG. 3.
Alkaline phosphatase activity at different zebrafish embryo developmental stages: blastula (A), gastrula (B), and larvae (C). Embryonic cells are alkaline phosphatase positive throughout development, regardless of their differentiation status. Color images available online at www.liebertonline.com/zeb
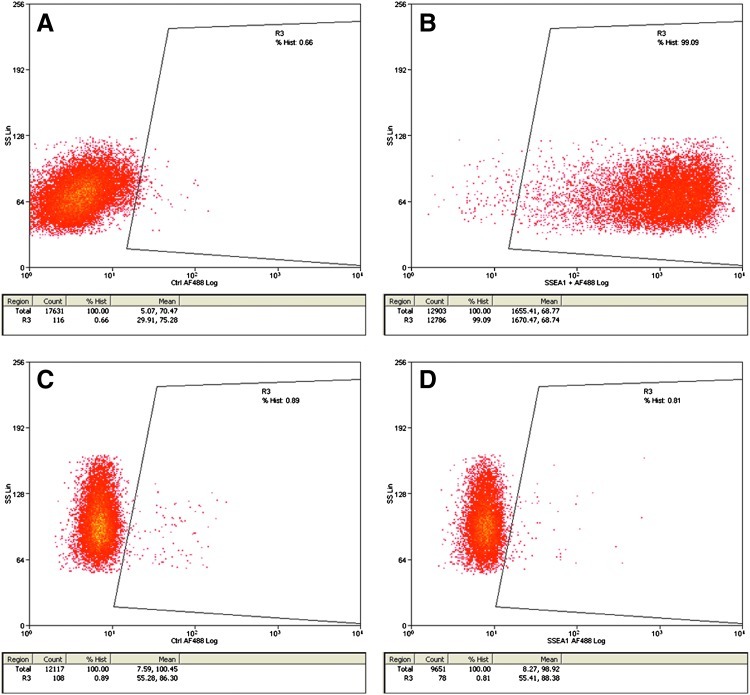
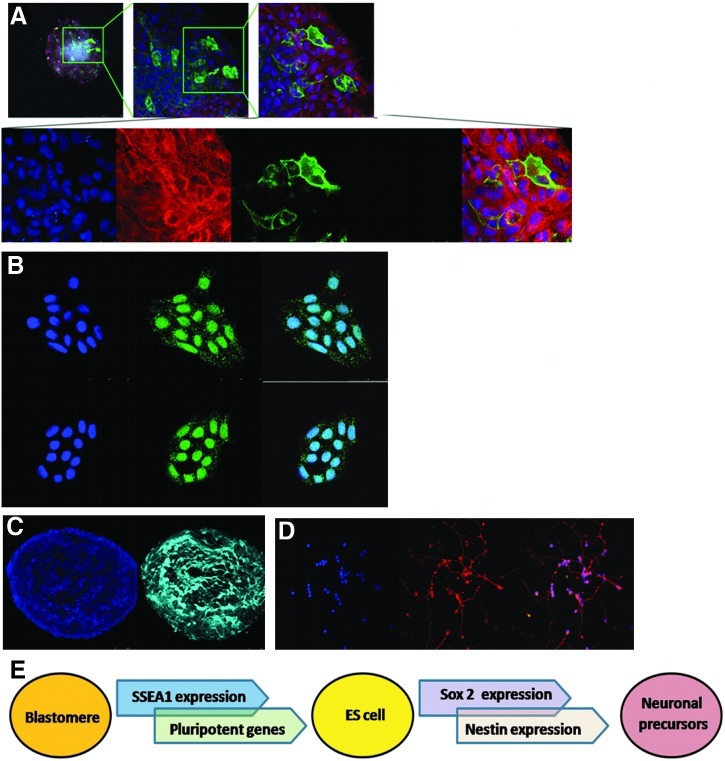
Regarding SSEA1, the protein was not detected by immunohistochemistry in zebrafish embryos at the oblong stage (when transient embryonic cell cultures were derived). To detect whether only a small percentage of blastomeres were positive for SSEA1 and they were simply not properly detected, we performed a FACS analysis using mouse ES cells as a positive control (Fig. 4). Our results showed, once again, the absence of this protein in the embryos (Fig. 4). However, after 10 days in culture (Fig. 5), some of these cells started to express SSEA1 (Fig. 6). Pou5f1 was clearly detected in zebrafish embryos at the oblong stage (Fig. 2), although no detection was observed in transient ES cell cultures (Fig. 6A). Sox2 was detected in neither embryos nor embryonic cells in culture, although it could be detected once some of the cells in these cultures started differentiating to neuronal precursors (Fig. 6B), followed by the expression of nestin (Fig. 6C), and after few more days, neurites were present in those cells (Fig. 6D).
FIG. 4.
Stage-specific embryonic antigen 1 (SSEA1) analyzed by FACS in mouse embryonic stem (ES) cells (A, B) and zebrafish blastomeres (C, D). Positive cells are present inside the marked area. Negative controls show no positive cells inside the area (A, C). Mouse ES cells are SSEA positive (B), but zebrafish blastomeres are negative (D). Color images available online at www.liebertonline.com/zeb
FIG. 5.
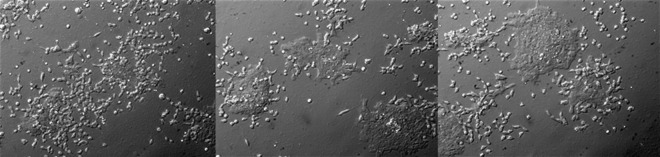
Seven-day transient cultures of zebrafish embryonic cells using two inhibitors (MEK and glycogen synthase kinase 3).
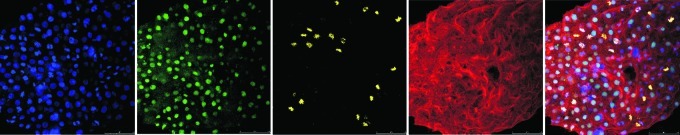
FIG. 6.
(A) SSEA1-positive cells (green) in transient ES cell cultures. All the cells are negative for OCT4 (red) and SOX2 (light blue). Cell nuclei are shown in blue (DAPI). (B) SOX2-positive cell colonies (DAPI, blue; SOX2, green; merged image). (C) Nestin-positive colonies (DAPI, blue; nestin, light blue). (D) Neuronal precursors. Nuclei are shown in blue (DAPI) and neurites in red (merged image). (E) Diagram showing markers that define the transition between blastomeres, ES cells, and neuronal precursors. Color images available online at www.liebertonline.com/zeb
Discussion
Zebrafish are emerging as one of the most useful animal models in science; however, zebrafish ES cell culture is not yet fully established. Some authors have tried to establish these cultures,13 and despite the publications on this matter, it is highly probable that these lines, which are referred to as ES cells, are in fact only blastula-derived lines.6 Here, we define a simple media for transient zebrafish embryonic cell culture, to use these cultures as a tool to study how pluripotency evolves when pluripotent embryonic cells are plated and cultured. It is known that ES cells can be kept in a ground state by addition of small molecule inhibitors for GSK3 and an inhibitor of the Fgf pathway.14 Inhibiting these two pathways also efficiently converts partially reprogrammed cell lines into fully reprogrammed iPS cells.15 We used these two inhibitors and achieved a transient equilibrium allowing cell proliferation without differentiation (Fig. 5). Indeed, we have observed that Mek i produces a blockade of differentiation, but affects cell growth and viability, and the addition of GSK3 i provides better conditions for ES cell self-renewal. However, this equilibrium is very unstable and it is commonly observed that pluripotency is inversely proportional to growth and viability. The successful establishment of these transient cultures, together with the use of embryonic cells, allowed us to study the pluripotency markers that are expressed in these cells and hypothesize their significance in this species. We consider that one of the problems in the establishment of zebrafish embryonic cell cultures is the lack of knowledge about these pluripotency markers, which necessitates the use of mammalian embryonic cell markers as zebrafish markers.
First, we studied the expression of pluripotency-associated markers in zebrafish blastomeres and adult somatic cells by quantitative PCR. Only 4 of the 9 studied genes (pou5f1, klf4, sall 4, and hsp60) showed higher expression in blastomeres than in adult somatic cells. Two of these genes (pou5f1 and klf4) are commonly used in reprogramming experiments16 and their upregulation in zebrafish embryonic cells was an expected result. It has been shown that the function of pou5f1 proteins is conserved between mouse and Xenopus and they regulate similar genes in ES cells and Xenopus embryos.17 It is thought that the ability of oct4 to maintain ES cell pluripotency is derived from the ancestral function of this class of proteins to maintain multipotency during early vertebrate development.17 Regarding hsp60, Qin and colleagues18 demonstrated that it was upregulated in regenerating retina. This gene, like hspa9 (heat shock 70-kDa protein 9), could possibly be required for rapid cell proliferation as a molecular chaperone.19
However, it is interesting that sox2, one of the key players in reprogramming, was expressed in adult somatic cells rather than in embryonic cells. In previous studies, we demonstrated by knockdown experiments in the zebrafish regenerating fin that sox2 is only needed for outgrowth of the blastema after 3 days, contrary to pou5f1, which is required for very early stages of fin blastema regeneration. As outgrowth requires innervation to proliferate and replace structures, we speculated a possible role for sox2 in specifying early neural precursor cells that contribute to the innervation of the regenerate.20 A role for sox2 in neurogenesis has been already described in other species and in mouse ES cells.21 Further, in the present study, we have observed that sox2 is expressed in blastula cell colonies that subsequently give rise to neuronal precursors (Fig. 6B–D). Therefore, we can conclude that in this case, the role of sox2 in neurogenesis is more important than its implication in pluripotency, and it cannot be used as a pluripotency marker in zebrafish.
Regarding the relative gene expression throughout development (Fig. 1B), we also observed that only pou5f1 has a peak of expression at the oblong stage. This stage is commonly used to derive transient embryonic cell cultures and it has been demonstrated that these blastula-derived cells are pluripotent because they are able to contribute toward a chimeric animal and the germline.4 The presence of the protein in zebrafish blastomeres at the oblong stage (when zygotic expression has already started) was confirmed by immunohistochemistry (Fig. 2).
SSEA1 is a mouse cell pluripotency marker that has also been used in zebrafish.6 In the present study, we observed that blastomeres are negative for this marker (Fig. 4). However, once in culture, some cells in the colonies become SSEA1 positive (Fig. 6A). On the other hand, we have observed that in zebrafish, sox2 plays an important role in neuronal differentiation, but not in pluripotency. We have observed that sox2 expression precedes nestin expression (Fig. 6B, C), a well-known neuronal precursor marker. Neurite formation was observed when these nestin-positive cells were kept in culture (Fig. 6D). These observations are in concordance with our gene expression studies, which did not show sox2 expression in embryonic cells (Fig. 1A). With all these data, it would be interesting to test whether SSEA1 is a marker that precedes expression of pluripotency genes in a zebrafish embryonic cell colony in the same way that sox2 precedes nestin expression in those colonies that have started differentiating to neurons (Fig. 6E). This phenomenon observed in zebrafish is parallel to mammalian cell reprogramming. In the reprogramming process, which could take 1–2 weeks, one of the upregulated early markers is ssea1. The ssea1-expressing cells then activate other pluripotency-associated genes, such as oct4, sox2, nanog, and tert, only late in the process.22
We have tried to go a step further in understanding zebrafish pluripotency markers by identifying markers that define cell populations prior to and during transition periods (blastomeres/embryonic cell cultures/differentiated cells), which we consider to be a crucial matter in the establishment of bona fide zebrafish ES cell cultures. Further studies about the sequence in which zebrafish genes are switched on and off during transient embryonic culture conditions and during differentiation could shed more light on these processes and also be a powerful tool for the monitoring of these cultures.
Acknowledgments
Work in the laboratory of J.C.I.B. was supported by funds from Fundacion Cellex, CIBER, and MICINN. The authors thank Ramón y Cajal program RYC-2008-02339 and MICINN AGL2009-06994. The authors also thank Carme Fabregat, Marina Raya, Marc Permanyer, Dr. Laura Batlle-Morera, J. Miguel Vaquero, and CMRB bioimagen platform staff for their technical assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Meng X. Noyes MB. Zhu LJ. Lawson ND. Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyon Y. McCammon JM. Miller JC. Faraji F. Ngo C. Katibah GE, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma C. Fan L. Ganassin R. Bols N. Collodi P. Production of zebrafish germ-line chimeras from embryo cell cultures. Proc Natl Acad Sci USA. 2001;98:2461–2466. doi: 10.1073/pnas.041449398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan L. Crodian J. Liu X. Alestrom A. Alestrom P. Collodi P. Zebrafish embryo cells remain pluripotent and germ-line competent for multiple passages in culture. Zebrafish. 2004;1:21–27. doi: 10.1089/154585404774101644. [DOI] [PubMed] [Google Scholar]
- 5.Fan LC. Moon J. Crodian J. Collodi P. Homologous recombination in zebrafish ES cells. Transgenic Res. 2006;15:21–30. doi: 10.1007/s11248-005-3225-0. [DOI] [PubMed] [Google Scholar]
- 6.Xing JG. Lee LEJ. Fan LC. Collodi P. Holt SE. Bols NC. Initiation of a zebrafish blastula cell line on rainbow trout stromal cells and subsequent development under feeder-free conditions into a cell line, ZEB2J. Zebrafish. 2008;5:49–60. doi: 10.1089/zeb.2007.0512. [DOI] [PubMed] [Google Scholar]
- 7.Shamblott MJ. Axelman J. Wang S. Bugg EM. Littlefield JW. Donovan PJ, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brambrink T. Foreman R. Welstead GG. Lengner CJ. Wernig M. Suh H. Jaenisch R. Sequential expression of pluripotent markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onichtchouk D. Geier F. Polok B. Messerschmidt D. Mössner R. Wendik B, et al. Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Sys Biol. 2010;6:354. doi: 10.1038/msb.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuda Y. Ogura E. Kondoh H. Kamachi Y. B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet. 2010;6:e1000936. doi: 10.1371/journal.pgen.1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel CB. Ballard WW. Kimmel SR. Ullmann B. Schilling TF. Stages of embryonic development of the zebrafish. Dev dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 12.Gibson UE. Heid CA. Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 13.Fan L. Collodi P. Klimanskaya I. Robert L. Zebrafish embryonic stem cells. In: Klimanskaya I, editor; Lance RP, editor. Methods in Enzymology. Vol. 418. San Diego, CA: Academic Press; 2006. pp. 64–77. [DOI] [PubMed] [Google Scholar]
- 14.Ying Q-L. Wray J. Nichols J. Batlle-Morera L. Doble B. Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva J. Barrandon O. Nichols J. Kawaguchi J. Theunissen TW. Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okita K. Nakagawa M. Hyenjong H. Ichisaka T. Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 17.Morrison GM. Brickman JM. Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development. 2006;133:2011–2022. doi: 10.1242/dev.02362. [DOI] [PubMed] [Google Scholar]
- 18.Qin Z. Barthel LK. Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci USA. 2009;106:9310–9315. doi: 10.1073/pnas.0811186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshinari N. Ishida T. Kudo A. Kawakami A. Gene expression and functional analysis of zebrafish larval fin fold regeneration. Dev Biol. 2009;325:71–81. doi: 10.1016/j.ydbio.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Christen B. Robles V. Raya M. Paramonov I. Izpisua Belmonte JC. Regeneration and reprogramming compared. BMC Biol. 2010;8:5. doi: 10.1186/1741-7007-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S. Choo ABH. Nai-Dy W. Heng-Phon T. Oh SKW. Knockdown of Oct-4 or Sox-2 attenuates neurogenesis of mouse embryonic stem cells. Stem Cells Dev. 2007;16:413–420. doi: 10.1089/scd.2006.0099. [DOI] [PubMed] [Google Scholar]
- 22.Stadtfeld M. Maherali N. Breault DT. Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]