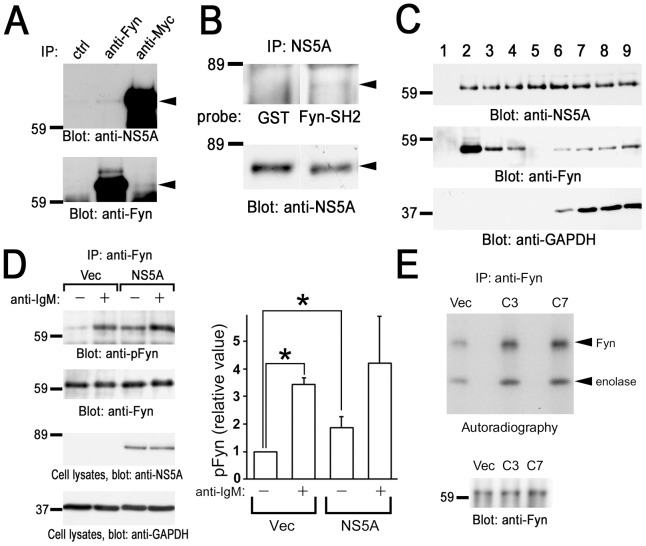
Figure 4. Association with NS5A increases the kinase activity of Fyn in B cells.
(A) Endogenous interaction of Fyn with NS5A in BJAB cells. Cells were solubilized in the lysis buffer containing 0.5% Nonidet P-40. Detergent-soluble lysates from BJAB cells expressing Myc-His-NS5A (clone 7) were subjected to immunoprecipitation with anti-Fyn or anti-Myc antibodies. Protein interactions between NS5A and Fyn were analyzed by the immunoblotting with anti-NS5A mAb and anti-Fyn antibody, respectively. (B) Anti-Myc immunoprecipitates were separated by SDS-PAGE and subjected to far western analysis with GST or GST-Fyn-SH2 (GST-Fyn-SH2) (upper panel), and immunoblotting analysis with anti-NS5A mAb (lower panel). (C) Cell homogenates were fractionated by sucrose density gradient centrifugation. Proteins from these fractions were separated by SDS-PAGE and analyzed with immunoblotting with anti-NS5A mAb, anti-Fyn, and anti-GAPDH antibodies. (D) Control cells (Vec) and cells expressing Myc-His-NS5A (clone 7) were unstimulated (−) or stimulated (+) with anti-IgM mAb. Anti-Fyn immunoprecipitates (IP) were separated by SDS-PAGE and analyzed by immunoblotting with anti-phospho-Src family (Tyr416) antibody recognizing autophosphorylated Fyn (pFyn) and anti-Fyn antibody. Detergent-soluble lysates were separated by SDS-PAGE and analyzed by immunoblotting with anti-NS5A and anti-GAPDH mAbs. Densitometry analysis was performed on three experiments representative of Fig. 4D. Levels of pFyn were normalized to their respective total Fyn protein. The fold changes of pFyn are shown relative to unstimulated control cells. Data represent the mean ± SD of three independent experiments. *, P<0.05. (E) Anti-Fyn immunoprecipitates (IP) from control cells (Vec), cells expressing Myc-His-NS5A clone 3 (C3) and clone 7 (C7) were subjected to in vitro kinase assay using enolase as an exogenous substrate. Radioactive proteins were separated by SDS-PAGE and visualized by autoradiography. Immunoprecipitated Fyn was analyzed by immunoblotting. Molecular sizing markers are indicated at left in kilodalton. The results were representative of three independent experiments. Similar results were obtained when another line was examined.