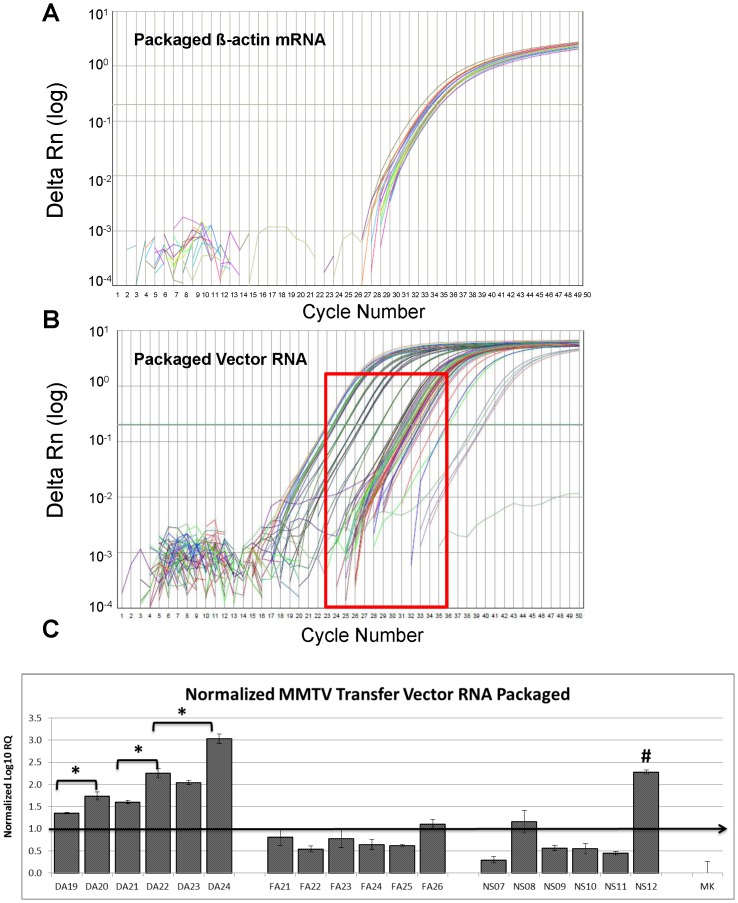
Figure 5. Relative packaging efficiency of MMTV transfer vector RNAs into the pseudotyped MMTV particles.
Real time PCR analysis of (A) packaged β-actin RNA into VSV-G-Env-pseudotyped MMTV particles and (B) packaged transfer vector RNAs into the VSV-G-Env-pseudotyped MMTV particles expressed as ΔRn verses cycle number. ΔRn is the target gene-specific fluorescence signal (FAM for MMTV-specific and VIC for ß-actin-specific sequences) normalized to the signal for the internal passive control, ROX (Normalized Reporter or Rn) from which the baseline target fluorescence has been subtracted (ΔRn = Normalized Reporter (Rn) - baseline). (C) Relative RNA packaging efficiencies for each of the mutant transfer vector RNA after normalization with β-actin and luciferase expression. The box in panel B highlights the wide range of threshold cycle (Ct) values observed for each transfer vector in comparison to the similar amounts of β-actin packaged into the viral particles. The arrow in panel C highlights the threshold value of detection. MK, Mock, cells transfected with packaging construct, JA10 + the VSV-G-Env expression plasmid, MD.G + luciferase-expression vector, pGL3 except the transfer vector. RQ, Relative Quantification in log10 units. *, statistically significant differences between constructs are shown by the brackets (p<0.01). #, statistically significant difference between NS12 and DA24 (p<0.01). The primers/probe for detecting the transfer vectors were designed within the U5 region of the MMTV LTR, a region common to all transfer vector RNAs. Each sample was tested in triplicates with MMTV- and β-actin-specific probes and primers in panel B, while the β-actin samples shown in panel A were tested in duplicates, as described in Materials and Methods.