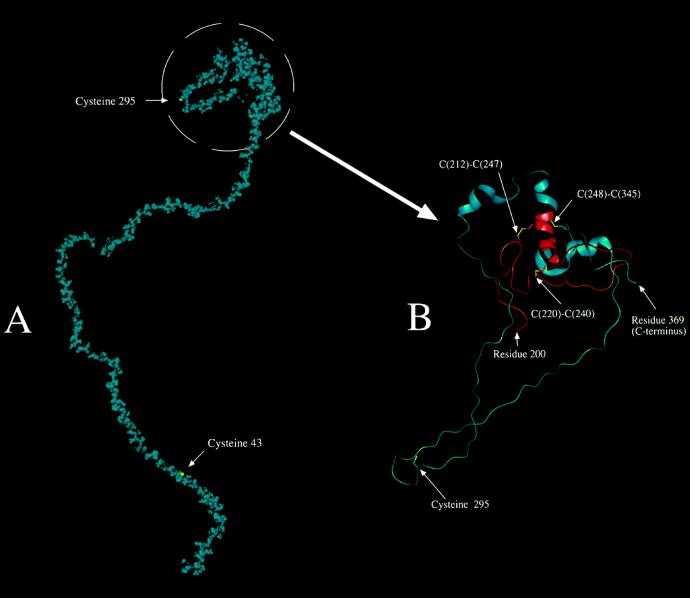
Figure 6.
Computer-generated molecular model of the 42K subunit. A, Entire 42K LMW-GS shown in space-filling form (van der Waals's radii for atoms) with all atoms shown in blue, except the sulfur atoms of Cys or cystine side chains, which are shown in yellow. B, Simplified model of the region containing the intramolecular disulfide linkages and Cys-295, which presumably forms one of two intermolecular disulfide cross-linkages. Residues 200 to 369 of the 42K subunit are shown in protein cartoon format, which displays the α-helical structure as a helical ribbon. There is also a specific display of selected side chains in a licorice (stick) bond format. The sulfur atoms of the Cys and cystine residues are shown in yellow. The main polypeptide chain is shown in red for residues 200 to 248 and in blue from there on to the C-terminal end at residue 369. The numbers of connected (intramolecular) Cys residues are shown.