Abstract
3-Iodothyronamine (T1AM) is an endogenous thyroid hormone derivative with unknown biosynthetic origins. Structural similarities have led to the hypothesis that T1AM is an extrathyroidal metabolite of T4. This study uses an isotope-labeled T4 [heavy-T4 (H-T4)] that can be distinguished from endogenous T4 by mass spectrometry, which allows metabolites to be identified based on the presence of this unique isotope signature. Endogenous T1AM levels depend upon thyroid status and decrease upon induction of hypothyroidism. However, in hypothyroid mice replaced with H-T4, the isotope-labeled H-T3 metabolite is detected, but no isotope-labeled T1AM is detected. These data suggest that T1AM is not an extrathyroidal metabolite of T4, yet is produced by a process that requires the same biosynthetic factors necessary for T4 synthesis.
Thyroid hormone (TH) is synthesized in the thyroid gland predominantly as T4. The first step in the biosynthesis of T4 involves concentration of dietary iodide in the thyroid gland by the sodium-iodide symporter (NIS), which is followed by thyroperoxidase (TPO)-catalyzed iodination of tyrosine residues within the thyroglobulin protein. T4 is a prohormone that is secreted from the thyroid gland and undergoes enzymatic outer ring deiodination to form T3. T3 is the active form of TH, and it binds to the TH nuclear receptors to regulate transcription of TH responsive genes (1). TH-mediated gene transcription affects a variety of physiological processes, including cardiac function, metabolism, bone turnover, body temperature, and cholesterol levels (2). Although most effects of TH are transcriptionally mediated, there are some rapid effects that are thought to be transcriptionally independent (3, 4).
In addition to outer ring deiodination, T4 is deiodinated on the inner ring to form rT3, a metabolite with unknown physiological significance. Inner ring deiodination of either T4 or T3 is thought to be a mechanism for deactivating TH (5), yet deiodination pathways sequentially produce diiodo- and monoiodothyronines (6, 7). Additional metabolic pathways of TH include conjugation with either a sulfate or a glucuronic acid (8). T4 and T3 can undergo oxidative deamination of the alanine side chain to form the thyroacetic acid derivatives, Tetrac and Triac, both of which have biological activity. Triac has thermogenic effects (9), whereas Tetrac inhibits T4 actions initiated at the plasma membrane (10). This extensive metabolism of TH beyond direct activation and deactivation of T3 indicates that TH metabolism is critical for regulating hormone action.
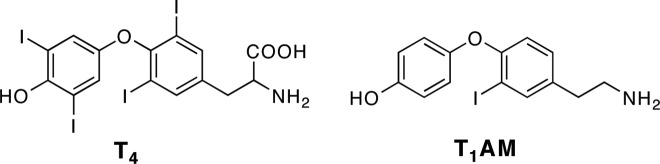
3-Iodothyronamine (T1AM) is an endogenous derivative of TH present in rat, mouse, hamster, and human, with acute pharmacological effects (11–15). A single pharmacological dose of T1AM results in hypothermia, bradycardia, a shift in the respiratory quotient, hyperglycemia, and hypoinsulinemia within minutes to a few hours (11, 13, 16). Structural similarities between T4 and T1AM, including the presence of iodine and the biaryl ether carbon skeletons (Fig. 1), suggest that T1AM is a metabolite of T4. T1AM is sulfated and deiodinated in vitro and oxidatively deaminated in vivo (17–19), indicating that it is a substrate for the same metabolic pathways that act on TH. A hypothesized biosynthetic pathway for T1AM involves deiodination and decarboxylation of TH. If decarboxylation of TH occurs, this would represent a previously uncharacterized branch of TH metabolism and signaling.
Fig. 1.
Structures of T4 and T1AM.
Several analytical methods have been developed to measure T1AM levels, including an immunoassay and liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays (12, 14, 20, 21). The advantage of LC-MS/MS over an immunoassay is that additional structural information is obtained. We have recently synthesized an isotope-labeled T4, 13C9-15N-T4 (Hackenmueller, S. A., and T. S. Scanlan, Synthetic Communications, in press). 13C9-15N-T4 [heavy-T4 (H-T4)] is 10 atomic mass units heavier than unlabeled T4, creating a unique mass signature that is detectable by mass spectrometry. This mass signature allows for H-T4 and any metabolite arising from H-T4 to be distinguishable from unlabeled T4 and metabolites. In this study, we use the labeled H-T4 to test the hypothesis that T1AM is a metabolite of T4.
Materials and Methods
Animals
Male C57Bl/6J mice, 8- to 10-wk-old, were housed four to five per cage in a climate-controlled room with a 12-h light, 12-h dark cycle. All animals had ad libitum access to food and water. Experimental protocols were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Oregon Health and Science University Institutional Animal Care and Use Committee.
Chemicals
For use with animals, potassium perchlorate (KClO4) was purchased from MP Biomedicals (Solon, OH). Methimazole (MMI) was purchased from Acros Organics (Pittsburgh, PA). T4 was purchased from Sigma (St. Louis, MO), and H-T4 was synthesized as described elsewhere (Hackenmueller, S. A., and T. S. Scanlan, Synthetic Communications, in press). Human serum deficient in T4 and T3 was purchased from Sigma.
Hormone manipulations
To determine the length of treatment necessary to induce hypothyroidism, mice were treated with 0.1% MMI and 0.2% KClO4 in drinking water for 2, 4, or 6 wk (22). Blood was collected and allowed to clot on ice for at least 30 min before centrifugation at 6000 × g. Sera were removed and stored at −80 C until further use. Total serum T4 was measured using a Coat-A-Count Total T4 RIA kit (Siemens Healthcare Diagnostics, Deerfield, IL).
For T4 replacement studies, mice were pretreated for 2 wk and subsequently maintained on MMI and KClO4 in drinking water for the duration of hormone replacement. T4 or H-T4 was dissolved in 0.01 n NaOH in sterile saline (0.9% NaCl; Hospira, Lake Forest IL) and administered as once daily sc injections (23). T4 was administered once daily for 2–5 wk to determine the time necessary to establish steady-state serum hormone levels. H-T4 was injected once daily for 3 wk to reestablish euthyroid hormone levels. Serum was collected as described above, and tissues, including liver, heart, brain, and kidney, were frozen immediately on dry ice and stored at −80 C until used for hormone extraction. Total serum T4 levels after hormone replacement were determined using the RIA kit described above.
T1AM, T3, and T4 extraction from liver
T1AM, T3, and T4 were extracted from mouse livers following a similar protocol to what has been previously described (21). Frozen livers were thawed, weighed, and homogenized in cold homogenization buffer [1 ml per 1 g of tissue, 154 mm NaCl, and 10 mm NaH2PO4 (pH 7.4)] with 30 passes in a Potter-Elvehjem tissue homogenizer attached to a Glas-Col motor drive. Homogenates were centrifuged at 4000 × g for 15 min. Supernatants were collected and transferred to clean 15-ml polypropylene tubes, and 2.5 pmol d4-T1AM, and 25 pmol each of 13C6-T3 and 13C6-T4 were added as internal standards. Additional 60 mg of NaCl were added per 1 ml of supernatant, and the samples were incubated at room temperature for 1 h. Acetone was added to each sample to precipitate proteins at a ratio of 2:1 acetone:supernatant volume, and samples were vortexed and left on ice for 30 min. Samples were centrifuged at 2000 × g for 15 min, and supernatants were evaporated in an Eppendorf Concentrator Plus 5301 evaporator (Eppendorf, Hamburg Germany) to remove acetone. Potassium acetate buffer [1 ml of 0.1 m (pH 4.0)] was added to each sample before solid phase extraction (SPE).
Bond-Elut Certify SPE cartridges (130 mg, 3 ml; Varian, Inc., Middleburg, The Netherlands) were preconditioned by gravity with 2 ml of methylene chloride/isopropanol (75:25 by volume), followed by 2 ml of methanol and 2 ml of potassium acetate buffer. Samples in potassium acetate buffer were added to the SPE cartridges, and the cartridges were washed sequentially with 3.5 ml of water, 1.6 ml of 0.1 m HCl, 7 ml of methanol, and 3.5 ml of methylene chloride/isopropanol (75:25). T1AM was eluted with 2 ml of methylene chloride/isopropanol/ammonium hydroxide (70:26.5:3.5 by volume) in clean borosilicate glass tubes. Eluates were transferred to Eppendorf tubes and evaporated to dryness in the Eppendorf evaporator described previously. Dried residues were redissolved in 100 μl of 1:1 methanol:0.1 m HCl before injection for LC-MS/MS analysis.
LC-MS/MS analysis
Samples were analyzed by LC-MS/MS using previously established selected reaction monitoring method (21). A 200 series HPLC system (PerkinElmer, Boston, MA), constituted by a binary micropump system, a column oven, and an autosampler, was coupled to an API 4000 triple quadrupole mass spectrometer (Applied Biosystems-MDS Sciex, Concord, Ontario, Canada) with a Turbo-V IonSpray source operated in the positive mode. Samples were injected onto a Gemini C18 2 × 50 mm, 3-μm particle size column (Phenomenex, Torrance, CA). Buffer A (0.1% formic acid in water) and buffer B (0.1% formic acid in methanol/acetonitrile) (1:4) were used in the following gradient: 5–100% A from 0 to 5 min, 100% A from 5 to 6 min, and equilibrate 6 to 9 min. IonSpray voltage was 5.25 kV, gas source 1 was 70, gas source 2 was 55, turbo temperature was 650 C, and collision-activated dissociation gas pressure was 5.7 mPa. Declustering potential, collision energy, and collision exit potential (CXP) parameters for each selected reaction monitoring ion pair monitored are listed in Table 1.
Table 1.
MS/MS instrument parameters
| Analyte | ID | Retention time (min) | Q1 (m/z) | Q3 (m/z) | CE | DP | CXP |
|---|---|---|---|---|---|---|---|
| T1AM | Known analyte | 2.4 | 356 | 339 | 18 | 53 | 10 |
| 212 | 28 | 53 | 18 | ||||
| 195 | 36 | 53 | 17 | ||||
| d4-T1AM | Internal standard | 2.4 | 360 | 343 | 18 | 53 | 10 |
| 216 | 28 | 53 | 18 | ||||
| 199 | 36 | 53 | 17 | ||||
| H-T1AM | Predicted metabolite | 2.4 | 365 | 347 | 18 | 53 | 10 |
| 220 | 28 | 53 | 18 | ||||
| 203 | 36 | 53 | 17 | ||||
| T3 | Known analyte | 3.4 | 652 | 606 | 29 | 103 | 17 |
| 508 | 29 | 103 | 15 | ||||
| 479 | 47 | 103 | 14 | ||||
| 13C6-T3 | Internal standard | 3.4 | 658 | 612 | 29 | 103 | 17 |
| 514 | 29 | 103 | 15 | ||||
| 485 | 47 | 103 | 14 | ||||
| H-T3 | Predicted metabolite | 3.4 | 662 | 615 | 29 | 103 | 17 |
| 517 | 29 | 103 | 15 | ||||
| 488 | 47 | 103 | 14 | ||||
| T4 | Known analyte | 3.6 | 778 | 732 | 33 | 106 | 20 |
| 634 | 34 | 106 | 18 | ||||
| 605 | 51 | 106 | 16 | ||||
| 13C6-T4 | Internal standard | 3.6 | 784 | 738 | 33 | 106 | 20 |
| 640 | 34 | 106 | 18 | ||||
| 611 | 51 | 106 | 16 | ||||
| H-T4 | Predicted metabolite | 3.6 | 788 | 741 | 33 | 106 | 20 |
| 643 | 34 | 106 | 18 | ||||
| 614 | 51 | 106 | 16 |
DP, Declustering potential; CE, collision energy; CEP, collision exit potential; ID, identity; Q, quadrupole.
Statistics
Data are presented as mean ± sem. Data were analyzed by one-way ANOVA to determine statistical significance.
Results
Hypothyroid and hormone replacement mouse model
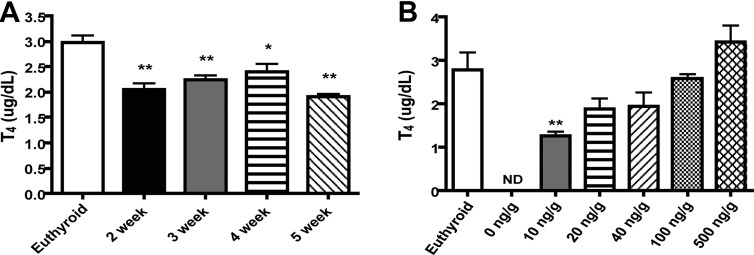
Multiple reports in the literature use a combination of MMI and ClO4− to induce hypothyroidism over the course of 4–14 wk (22, 24, 25). Because the half-life of T4 in mice is 13–18 h, we hypothesized that a period of treatment shorter than 4 wk may be sufficient to induce hypothyroidism (26). To test this, we conducted a time-course experiment with MMI and KClO4 administered in drinking water and monitored biweekly thyroid status as indicated by total serum T4. Wild-type euthyroid mice had serum T4 levels of 2.52 ± 0.32 μg/dl before inducing hypothyroidism with MMI and KClO4. After 2 wk of treatment with MMI and KClO4, total serum T4 levels were below the assay limit of detection (0.82 μg/dl). From these experiments, we concluded that 2 wk of MMI and KClO4 treatment was sufficient to induce hypothyroidism. For future experiments involving hormone replacement, mice were pretreated with MMI and KClO4 for 2 wk and maintained on MMI and KClO4 in drinking water throughout the duration of the experiments to inhibit endogenous TH synthesis.
Next, a time-course experiment was conducted to determine the length of time necessary to reestablish steady-state serum T4 in a hypothyroid mouse. Previous studies in the literature involving TH replacement in Pax8 knockout or Pax8/MCT8 double knockout mice use a dose of 20 ng/g daily, and thus, we used this as our initial replacement dose of T4 (23, 27). At 2–5 wk of T4 replacement, total serum T4 levels were significantly decreased with respect to euthyroid mice (Fig. 2A) but were not significantly different from each other. Based on these data, T4 serum levels reach steady-state within 2 wk, but the replacement dose of 20 ng/g was insufficient to reestablish euthyroid serum T4 levels. A previous report in the literature found that 3 wk of hormone replacement were necessary for the thyroid axis to reach steady state, as measured by TSH (28), prompting us to use a 3-wk hormone replacement duration (27). Because a 20 ng/g T4 replacement dose did not reestablish complete euthyroid serum T4 levels, a dose-response study was conducted. Hypothyroid mice were hormone replaced for 3 wk with T4 ranging in doses from 0–500 ng/g (Fig. 2B), which resulted in dose-dependent restoration of serum T4. We selected 100 ng/g T4 as a replacement dose for future studies, because this dose resulted in serum T4 levels closest to those of the euthyroid control.
Fig. 2.
Establishment of a hypothyroid and TH replacement mouse model. Total serum T4 levels were measured by RIA after treatment with 0.1% MMI and 0.2% KClO4 and replacement with 20 ng/g T4 for 2–5 wk (A) or replacement with 0–500 ng/g T4 for 3 wk (B); n = 3–5 per group; *, P < 0.05; **, P < 0.01. ND, Not detectable.
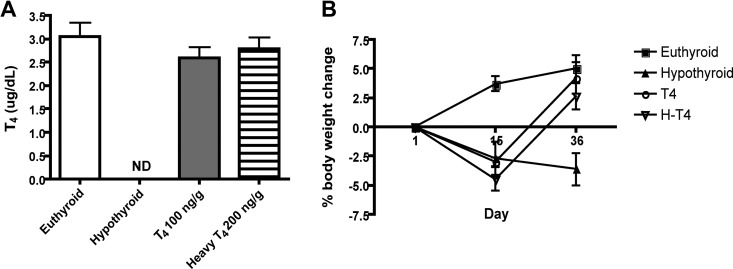
H-T4 replacement
In hypothyroid mice, replacement with 100 ng/g H-T4 did not restore serum T4 to euthyroid levels (data not shown). However, 200 ng/g H-T4 resulted in euthyroid serum T4 levels (2.79 ± 0.54 ng/dl) (Fig. 3A). Replacement with either 100 ng/g T4 or 200 ng/g H-T4 also normalized body weight after hypothyroid-induced weight loss (Fig. 3B). One interpretation of our results is that the T4 antibody in the RIA kit binds less efficiently to isotope-labeled H-T4 than to T4, resulting in artificially lower T4 serum concentrations in H-T4-replaced mice. To determine whether the difference in replacement doses between T4 and H-T4 was due to isotope effects on antibody binding, the response of T4 and H-T4 in the RIA kit was investigated. T4 and H-T4 were spiked into T4/T3-deficient human serum and analyzed by RIA. There was no difference in detection of H-T4 from T4 by the T4 antibody from 0 to 12.5 μg/dl (data not shown). This suggests that the difference in doses is not due to the presence of 13C isotopes but is likely due to a lack of bioequivalence between the T4 and H-T4 formulations, an issue that has been documented for different T4 preparations (29).
Fig. 3.
H-T4 replacement in a hypothyroid mouse model. A, Total serum T4 levels as measured by RIA after 2 wk of treatment with 0.1% MMI and 0.2% KClO4 and replacement with T4 or H-T4; n = 4–5 per group. B, Body weights of euthyroid, hypothyroid, T4, and H-T4-replaced mice. Hypothyroid, T4 and H-T4-replaced mice were treated with 0.1% MMI and 0.2% KClO4 starting on d 1 and continuing through d 36. At d 15, replaced mice began receiving daily injections of either T4 or H-T4; n = 4–8 per group. ND, Not detectable.
Endogenous T1AM levels in mouse liver
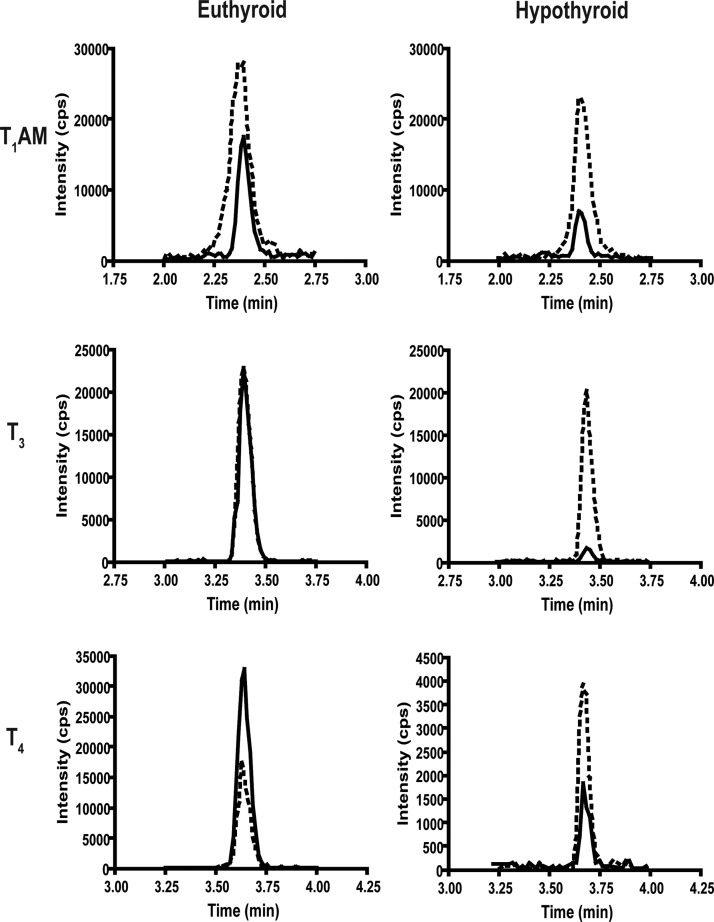
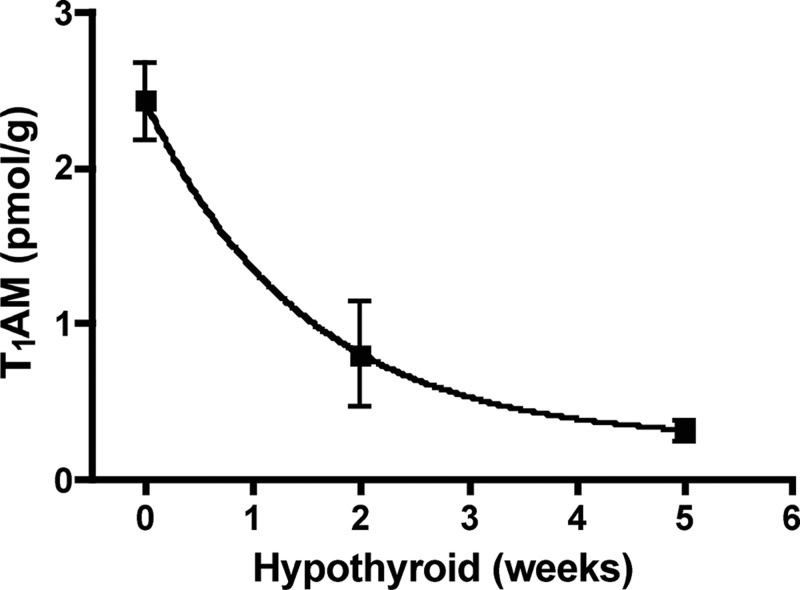
To test whether T1AM biosynthetic origins are related to thyroid status, we measured endogenous T1AM after induction of hypothyroidism. To date, the distribution of endogenous T1AM in mouse tissues has not been reported. The distribution of T1AM in rat tissues, where the greatest concentration of T1AM was present in liver, was used as a guide (21). For these studies, multiple tissues were initially analyzed to determine in which tissues T1AM is present, but ultimately, liver was used for analysis because this tissue contained the greatest concentration of T1AM in the mouse tissues analyzed. Detection of T1AM from other mouse tissues was not reproducible and thus not included in this analysis. Similar to the data reported for rat serum (21), mouse serum T1AM is lower than liver T1AM. T4 and T3 were monitored as experimental controls, because T4 and T3 levels are known to decrease in hypothyroidism. Endogenous T1AM from liver homogenates was analyzed by LC-MS/MS, and the chromatographic trace corresponding to the mass to charge ratio (m/z) transition of 356.2 to 339.1 shows a decrease in T1AM peak intensity after induction of hypothyroidism for 2 wk (Fig. 4). Ionization is sample dependent, and each sample is normalized by integrating peak areas with respect to the internal standards. As expected, T4 and T3 also decrease by over 90% upon induction of hypothyroidism. Due to sample-dependent variations in ionization efficiencies, to quantify T4, T3, and T1AM, peak areas were integrated with respect to the corresponding internal standard (d4-T1AM, 13C6-T3, or 13C6-T4). We integrated the peak areas of T1AM and d4-T1AM to determine the concentration of endogenous T1AM and found that liver T1AM concentration decreases time dependently with induction of hypothyroidism (Fig. 5).
Fig. 4.
Representative LC-MS/MS chromatogram for T1AM, T3, and T4 from euthyroid and 2-wk hypothyroid mouse livers. The m/z transitions represented are: T1AM, 356 to 339; d4-T1AM, 360 to 343; T3, 652 to 606; 13C6-T3, 658 to 612; T4, 778 to 732; and 13C6-T4, 784 to 738. Solid black line, Endogenous analyte; dashed line, internal standard. Representative traces of three samples analyzed for each group. cps, Counts per second.
Fig. 5.
T1AM levels decrease in mouse liver as a function of time of 0.1% MMI and 0.2% KClO4 treatment, as measured by LC-MS/MS; n = 3–5 per time point.
Metabolism of exogenous T4 and H-T4
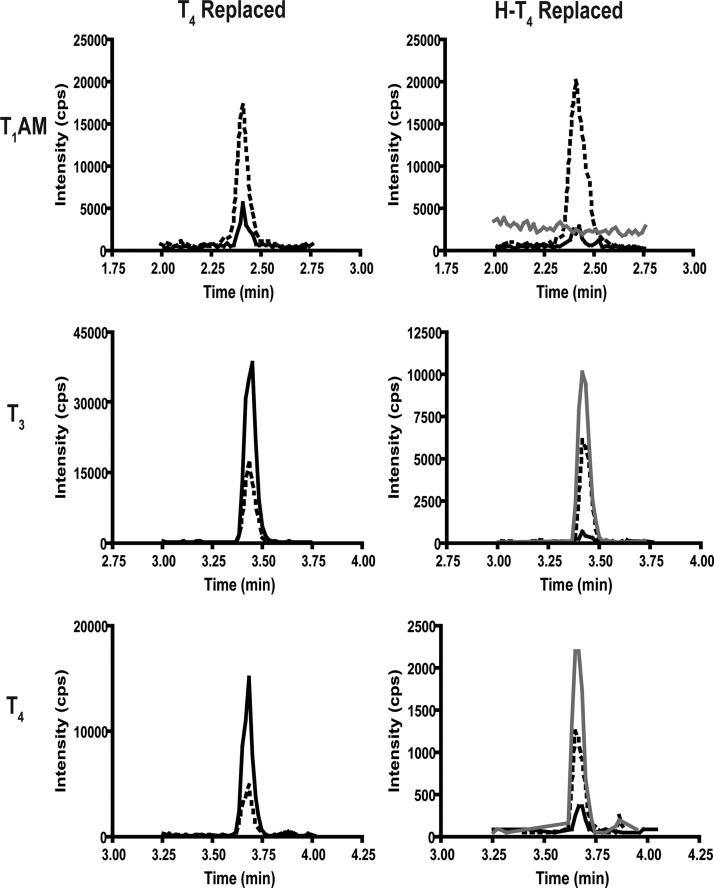
We next investigated whether T1AM is an extrathyroidal metabolite of T4 by measuring T1AM in hypothyroid mice that were hormone replaced with either T4 or H-T4. Homogenized livers were analyzed by LC-MS/MS for the presence of unlabeled and labeled (H-T1AM) forms of T1AM. T4, T3, and the corresponding heavy-labeled forms, H-T4 and H-T3, served as positive controls, because T3 is a known metabolite of T4. Representative chromatographic traces for T4 and H-T4-replaced mouse livers are shown in Fig. 6. The retention times of internal standards are used to additionally verify analytes, because the internal standards and corresponding unlabeled or heavy analytes coelute. In hypothyroid mice, replacement with T4 results in increased peak intensities for T4 and T3 relative to hypothyroid levels but no increase in T1AM peak intensity (Fig. 6). Replacement with H-T4 results in the detection of chromatographic peaks corresponding to H-T4 and H-T3 but no detectable chromatographic peak corresponding to H-T1AM.
Fig. 6.
Representative LC-MS/MS chromatogram for T1AM, T3, and T4 from T4 and H-T4-replaced mouse livers. The m/z transitions represented are: T1AM, 356 to 339; d4-T1AM, 360 to 343; H-T1AM, 365 to 347; T3, 652 to 606; 13C6-T3, 658 to 612; H-T3, 662 to 615; T4, 778 to 732; 13C6-T4, 784 to 738; and H-T4, 788 to 741. Solid black line, Unlabeled analyte (endogenous analyte for T1AM and H-T4-replaced group, and metabolite of exogenous T4 in T4-replaced group); dashed line, internal standard; gray line, heavy metabolite from H-T4. cps, Counts per second.
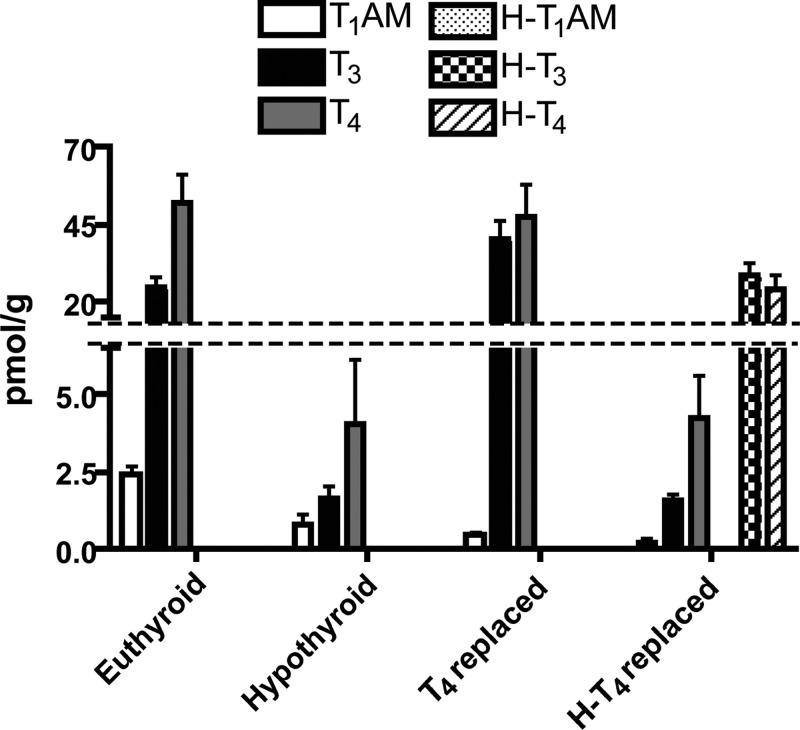
To quantify liver concentrations of unlabeled and labeled T4, T3, and T1AM, we integrated peak areas with respect to the corresponding internal standard in euthyroid, 2-wk hypothyroid, T4-replaced, and H-T4-replaced mice (Fig. 7). Inducing hypothyroidism decreases endogenous T4 and T3 92 and 93.6%, respectively (52.0 ± 16.0 to 4.1 ± 3.5 pmol/g for T4 and 25.0 ± 5.5 to 1.6 ± 0.7 pmol/g for T3). Replacement with T4 increases liver T4 to 91% and liver T3 to 161.6% of euthyroid liver concentrations. Replacement with H-T4 results in liver H-T4 and H-T3 of 46% (24.0 ± 8.2 pmol/g) and 114.4% (28.6 ± 6.8 pmol/g), respectively, whereas endogenous, unlabeled T4 and T3 remain decreased, at 4.2 ± 2.3 and 1.6 ± 0.3 pmol/g. Endogenous, unlabeled liver T1AM concentration decreases with hypothyroidism, from 2.4 ± 0.4 to 0.8 ± 0.6 pmol/g, and remains decreased upon replacement with either T4 (0.4 ± 0.1 pmol/g) or H-T4 (0.2 ± 0.1 pmol/g). No H-T1AM was detected from any mouse liver replaced with H-T4. Although liver T3 levels are increased after replacement with T4, this is unlikely to impact the final results, because T4 levels remain normal, and a lack of T1AM formation is also observed upon replacement with H-T4, which results in normal liver H-T3 levels.
Fig. 7.
Liver T4, T3, T1AM and H-T4, H-T3, and H-T1AM levels in euthyroid, hypothyroid, and hypothyroid mice with T4 or H-T4 replacement, as measured by LC-MS/MS. Inducing hypothyroidism for 2 wk decreases endogenous T4, T3, and T1AM. Replacement with T4 results in normal T4 and increased T3 with respect to euthyroid but no increase in T1AM with respect to hypothyroid. Replacement with H-T4 results in measurable H-T4 and H-T3, no increase in endogenous T4, T3 or T1AM, and no detectable H-T1AM. H-T4 and H-T3 were only detected in mice replaced with H-T4; n = 3 for euthyroid, hypothyroid, and H-T4 replaced; n = 2 for T4 replaced.
Discussion
T1AM is a recently discovered endogenous compound that contains the unique chemical signature of a TH, namely a biaryl ether core structure containing an iodine substituent. Like the TH T4 and T3, T1AM is found in various tissues and in circulation (21), and circulating T1AM is largely bound to lipoprotein particles (30). Recent advances in the quantitative analysis of T1AM using methods based on LC-MS/MS and T1AM-specific immunoassay suggest that endogenous T1AM is present in tissues (in the rat) and circulation (in humans) at levels that are comparable with those of total T4 (14, 21). In several rat tissues, including heart, liver, kidney, skeletal muscle, and stomach, T1AM is present at a greater concentration than T3, the major active metabolite of T4 (21). Although T1AM has been detected previously in mice, the distribution and quantitative analysis of T1AM in mice has not yet been studied with these improved methods.
Since its discovery, it has been proposed, if not assumed, that T1AM is produced as a metabolite of T4 by deiodination and decarboxylation, the hypothetical enzymatic steps required for conversion of T4 to T1AM. In support of this notion, plausible iodothyronamine deiodination pathways have been demonstrated in vitro (19). In addition, analysis of human serum samples showed higher levels of T1AM in samples from thyroid cancer patients receiving elevated hormone replacement doses of T4 to suppress TSH compared with samples from euthyroid control subjects [see Hoefig et al. (14)]. However, in this same study, no correlation was found between T1AM levels and either T4 or TSH levels, contrary to expectations were T4 to be a biosynthetic precursor of T1AM. Moreover, iodothyronine decarboxylation to iodothyronamines has not been demonstrated, and the well-studied aromatic amino acid decarboxylase, a somewhat nonspecific enzyme with respect to aromatic amino acid substrates, was recently shown to be unable to catalyze iodothyronine decarboxylation (31), suggesting that the enzymatic conversion of an iodothyronine to an iodothyronamine is not as straightforward as was originally thought. We have attempted here to address this question directly with a labeling experiment using a stable isotope-labeled form of T4 (H-T4) designed to afford a correspondingly labeled T1AM if T1AM were to arise from enzymatic processing of extrathyroidal T4. Detecting the H-T4 and its metabolites is done using LC-MS/MS, thus avoiding the use of radioactivity. Moreover, the labeled atoms of H-T4 reside in the thyronine skeleton and are not lost upon deiodination, as would be the case with commercially available outer ring [125I]-T4. Of note, a study designed similarly to ours was recently attempted and failed, because the investigators were unable to extract and detect endogenous T1AM by LC-MS/MS (32).
Our results show that endogenous liver T1AM concentration decreases significantly in hypothyroid vs. euthyroid mice, indicating a role for a functioning thyroid gland in the biosynthesis of T1AM. However, hypothyroid mice treated with H-T4 did not generate detectable levels of H-T1AM, whereas H-T3 was unequivocally detected. Taken together, these results indicate that T1AM biosynthesis can be inhibited by the antithyroid drugs MMI and KClO4 that target thyroid gland function by inhibiting TPO and the NIS, respectively (33, 34), yet does not appear to involve extrathyroidal deiodination and decarboxylation of T4, the previously favored and often hypothesized route of production of T1AM. The NIS and TPO targets of these antithyroid drugs implicate iodide transport and thyronine biosynthetic machinery in T1AM production that is generally regarded as being specific to the thyroid gland. These unexpected results lead to the conclusion that like T4, T1AM is either a direct product of the thyroid gland, or a product of another cell population that expresses NIS and TPO or homologs in functional form, or is a metabolite of another thyroid gland product that does not arise from extrathyroidal metabolism of T4.
At least three hypothetical thyroid gland-dependent pathways for T1AM biosynthesis can be considered, all of which are dependent upon thyroglobulin, the substrate for T4 biosynthesis. The first of these three involves extrathyroidal coupling of a phenol to monoiodotyrosine, a known by-product of thyroglobulin proteolysis that occurs in the T4 secretion process (35). The outer ring phenol could theoretically derive from tyrosine. However, the chemistry required for this putative coupling reaction is not presently known. The second possibility is that T1AM is secreted directly from the thyroid gland as a direct cleavage product of thyroglobulin. This putative pathway would begin with a thyroglobulin-bound 3-iodothyronine residue arising from TPO-mediated cross-coupling of monoiodotyrosine and tyrosine residues within thyroglobulin. Release of T1AM would then require an unknown proteolytic decarboxylation reaction or proteolysis followed by specific 3-iodothyronine decarboxylation occurring within the thyrocyte. The final possibility is that T1AM is a metabolite of a non-T4 thyroid gland product or a metabolite of T4 that is specifically generated within the thyroid gland. One potential candidate for such a T1AM precursor could be 3,5-diiodothyronine (3,5-T2) (36). To date, there is no reported direct evidence for outer ring deiodination of T3 in vivo to produce 3,5-T2 (20, 37), leaving open the possibility that like T1AM, 3,5-T2 is not an extrathyroidal metabolite of T4. If 3,5-T2 is not a simple deiodination product of circulating T4, then it most likely is produced from thyroglobulin-bound 3,5-T2 arising from TPO-catalyzed cross-coupling of 3,5-diiodotyrosine and tyrosine residues of thyroglobulin, respectively. Alternatively, if free T4 within the thyroid gland were converted to some other derivative via a reversible thyroid gland-specific biochemical process (e.g. N-methylation or acetylation), and this putative derivative was the direct biosynthetic precursor of T1AM, then a lack of labeled T1AM arising from labeled extrathyroidal T4 might also be expected to occur.
Although often thought of as thyroid gland specific, the dependence of T1AM synthesis on TPO and NIS does not preclude the involvement of an extrathyroidal de novo biosynthetic pathway. The expression of the NIS gene has been detected in numerous cultured skin cells (38), and both TPO and NIS mRNA expression has been detected in human skin samples (39). Additionally, a recent report documents that human fibrocytes express thyroglobulin and are able to take up and incorporate [125I] into the protein (40). The expression of these proteins in a cell population outside the thyroid gland creates the possibility that T1AM biosynthesis originates through a de novo pathway involving extrathyroidal iodination and cross-coupling of tyrosine residues.
Future studies are needed to distinguish between these possible routes or to discover alternative pathways from those proposed above. This work also points to a compelling need to reassess the unique chemistry that occurs within the thyroid gland. The powerful bioanalytical tools available today may reveal new chemical insights into the function of this important endocrine organ.
Acknowledgments
This work was supported by the National Institutes of Health Grant DK-52798 (T.S.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- H-T4
- Heavy-T4
- KClO4
- potassium perchlorate
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MMI
- methimazole
- m/z
- mass to charge ratio
- NIS
- sodium-iodide symporter
- SPE
- solid phase extraction
- 3,5-T2
- 3,5-diiodothyronine
- T1AM
- 3-iodothyronamine
- TH
- thyroid hormone
- TPO
- thyroperoxidase.
References
- 1. Cheng SY, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 3. Sun ZQ, Ojamaa K, Coetzee WA, Artman M, Klein I. 2000. Effects of thyroid hormone on action potential and repolarizing currents in rat ventricular myocytes. Am J Physiol Endocrinol Metab 278:E302–E307 [DOI] [PubMed] [Google Scholar]
- 4. Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ. 2005. Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146:2864–2871 [DOI] [PubMed] [Google Scholar]
- 5. St Germain DL, Galton VA, Hernandez A. 2009. Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology 150:1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burman KD, Strum D, Dimond RC, Djuh YY, Wright FD, Earll JM, Wartofsky L. 1977. A radioimmunoassay for 3,3′-L-diiodothyronine (3,3′T2). J Clin Endocrinol Metab 45:339–352 [DOI] [PubMed] [Google Scholar]
- 7. Corcoran JM, Eastman CJ. 1983. Radioimmunoassay of 3-L-monoiodothyronine: application in normal human physiology and thyroid disease. J Clin Endocrinol Metab 57:66–70 [DOI] [PubMed] [Google Scholar]
- 8. Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ. 2005. Alternate pathways of thyroid hormone metabolism. Thyroid 15:943–958 [DOI] [PubMed] [Google Scholar]
- 9. Medina-Gomez G, Calvo RM, Obregon MJ. 2008. Thermogenic effect of triiodothyroacetic acid at low doses in rat adipose tissue without adverse side effects in the thyroid axis. Am J Physiol Endocrinol Metab 294:E688–E697 [DOI] [PubMed] [Google Scholar]
- 10. Lin HY, Davis FB, Gordinier JK, Martino LJ, Davis PJ. 1999. Thyroid hormone induces activation of mitogen-activated protein kinase in cultured cells. Am J Physiol 276:C1014–C1024 [DOI] [PubMed] [Google Scholar]
- 11. Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. 2004. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med 10:638–642 [DOI] [PubMed] [Google Scholar]
- 12. DeBarber AE, Geraci T, Colasurdo VP, Hackenmueller SA, Scanlan TS. 2008. Validation of a liquid chromatography-tandem mass spectrometry method to enable quantification of 3-iodothyronamine from serum. J Chromatogr A 1210:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braulke LJ, Klingenspor M, DeBarber A, Tobias SC, Grandy DK, Scanlan TS, Heldmaier G. 2008. 3-Iodothyronamine: a novel hormone controlling the balance between glucose and lipid utilisation. J Comp Physiol B 178:167–177 [DOI] [PubMed] [Google Scholar]
- 14. Hoefig CS, Köhrle J, Brabant G, Dixit K, Yap B, Strasburger CJ, Wu Z. 2011. Evidence for extrathyroidal formation of 3-iodothyronamine in humans as provided by a novel monoclonal antibody-based chemiluminescent serum immunoassay. J Clin Endocrinol Metab 96:1864–1872 [DOI] [PubMed] [Google Scholar]
- 15. Galli E, Marchini M, Saba A, Berti S, Tonacchera M, Vitti P, Scanlan TS, Iervasi G, Zucchi R. 2012. Detection of 3-iodothyronamine in human patients: a preliminary study. J Clin Endocrinol Metab 97:E69–E74 [DOI] [PubMed] [Google Scholar]
- 16. Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. 2007. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest 117:4034–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pietsch CA, Scanlan TS, Anderson RJ. 2007. Thyronamines are substrates for human liver sulfotransferases. Endocrinology 148:1921–1927 [DOI] [PubMed] [Google Scholar]
- 18. Wood WJ, Geraci T, Nilsen A, DeBarber AE, Scanlan TS. 2009. Iodothyronamines are oxidatively deaminated to iodothyroacetic acids in vivo. Chembiochem 10:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piehl S, Heberer T, Balizs G, Scanlan TS, Smits R, Koksch B, Köhrle J. 2008. Thyronamines are isozyme-specific substrates of deiodinases. Endocrinology 149:3037–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piehl S, Heberer T, Balizs G, Scanlan TS, Köhrle J. 2008. Development of a validated liquid chromatography/tandem mass spectrometry method for the distinction of thyronine and thyronamine constitutional isomers and for the identification of new deiodinase substrates. Rapid Commun Mass Spectrom 22:3286–3296 [DOI] [PubMed] [Google Scholar]
- 21. Saba A, Chiellini G, Frascarelli S, Marchini M, Ghelardoni S, Raffaelli A, Tonacchera M, Vitti P, Scanlan TS, Zucchi R. 2010. Tissue distribution and cardiac metabolism of 3-iodothyronamine. Endocrinology 151:5063–5073 [DOI] [PubMed] [Google Scholar]
- 22. Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St Germain DL. 2007. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology 148:5680–5687 [DOI] [PubMed] [Google Scholar]
- 23. Christ S, Biebel UW, Hoidis S, Friedrichsen S, Bauer K, Smolders JW. 2004. Hearing loss in athyroid pax8 knockout mice and effects of thyroxine substitution. Audiol Neurootol 9:88–106 [DOI] [PubMed] [Google Scholar]
- 24. Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. 2007. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ueta CB, Olivares EL, Bianco AC. 2011. Responsiveness to thyroid hormone and to ambient temperature underlies differences between brown adipose tissue and skeletal muscle thermogenesis in a mouse model of diet-induced obesity. Endocrinology 152:3571–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Buul-Offers S, Hackeng WH, Schopman W. 1983. Thyroxine and triiodothyronine levels in Snell mice. Acta Endocrinol 102:396–409 [DOI] [PubMed] [Google Scholar]
- 27. Trajkovic-Arsic M, Müller J, Darras VM, Groba C, Lee S, Weih D, Bauer K, Visser TJ, Heuer H. 2010. Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology 151:5053–5062 [DOI] [PubMed] [Google Scholar]
- 28. Theodossiou C, Skrepnik N, Robert EG, Prasad C, Axelrad TW, Schapira DV, Hunt JD. 1999. Propylthiouracil-induced hypothyroidism reduces xenograft tumor growth in athymic nude mice. Cancer 86:1596–1601 [PubMed] [Google Scholar]
- 29. Henderson JD, Esham RH. 2001. Generic substitution: issues for problematic drugs. South Med J 94:16–21 [PubMed] [Google Scholar]
- 30. Roy G, Placzek E, Scanlan TS. 2012. ApoB-100-containing lipoproteins are major carriers of 3-iodothyronamine in circulation. J Biol Chem 287:1790–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoefig CS, Renko K, Piehl S, Scanlan TS, Bertoldi M, Opladen T, Hoffmann GF, Klein J, Blankenstein O, Schweizer U, Köhrle J. 2012. Does the aromatic L-amino acid decarboxylase contribute to thyronamine biosynthesis? Mol Cell Endocrinol 349:195–201 [DOI] [PubMed] [Google Scholar]
- 32. Ackermans MT, Klieverik LP, Ringeling P, Endert E, Kalsbeek A, Fliers E. 2010. An online solid-phase extraction-liquid chromatography-tandem mass spectrometry method to study the presence of thyronamines in plasma and tissue and their putative conversion from 13C6-thyroxine. J Endocrinol 206:327–334 [DOI] [PubMed] [Google Scholar]
- 33. Cooper DS. 1984. Antithyroid drugs. N Engl J Med 311:1353–1362 [DOI] [PubMed] [Google Scholar]
- 34. Van Sande J, Massart C, Beauwens R, Schoutens A, Costagliola S, Dumont JE, Wolff J. 2003. Anion selectivity by the sodium iodide symporter. Endocrinology 144:247–252 [DOI] [PubMed] [Google Scholar]
- 35. de la Vieja A, Calero M, Santisteban P, Lamas L. 1997. Identification and quantitation of iodotyrosines and iodothyronines in proteins using high-performance liquid chromatography by photodiode-array ultraviolet-visible detection. J Chromatogr B Biomed Sci Appl 688:143–149 [DOI] [PubMed] [Google Scholar]
- 36. Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. 2008. Metabolic effects of thyroid hormone derivatives. Thyroid 18:239–253 [DOI] [PubMed] [Google Scholar]
- 37. Pinna G, Brödel O, Visser T, Jeitner A, Grau H, Eravci M, Meinhold H, Baumgartner A. 2002. Concentrations of seven iodothyronine metabolites in brain regions and the liver of the adult rat. Endocrinology 143:1789–1800 [DOI] [PubMed] [Google Scholar]
- 38. Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, Chung JH, Giuliani C, Thornton M, Slugocki G, Tobin DJ. 2002. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol 119:1449–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cianfarani F, Baldini E, Cavalli A, Marchioni E, Lembo L, Teson M, Persechino S, Zambruno G, Ulisse S, Odorisio T, D'Armiento M. 2010. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol 130:93–101 [DOI] [PubMed] [Google Scholar]
- 40. Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, Smith TJ. 2012. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA 109:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]