Abstract
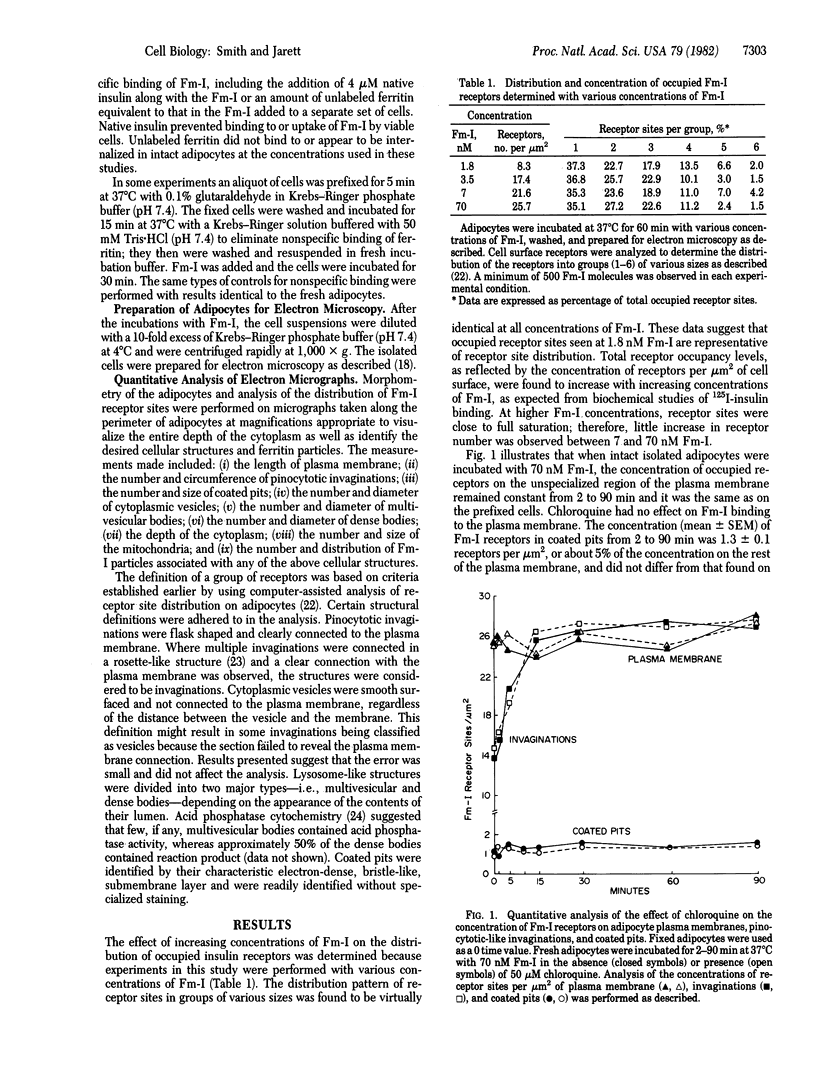
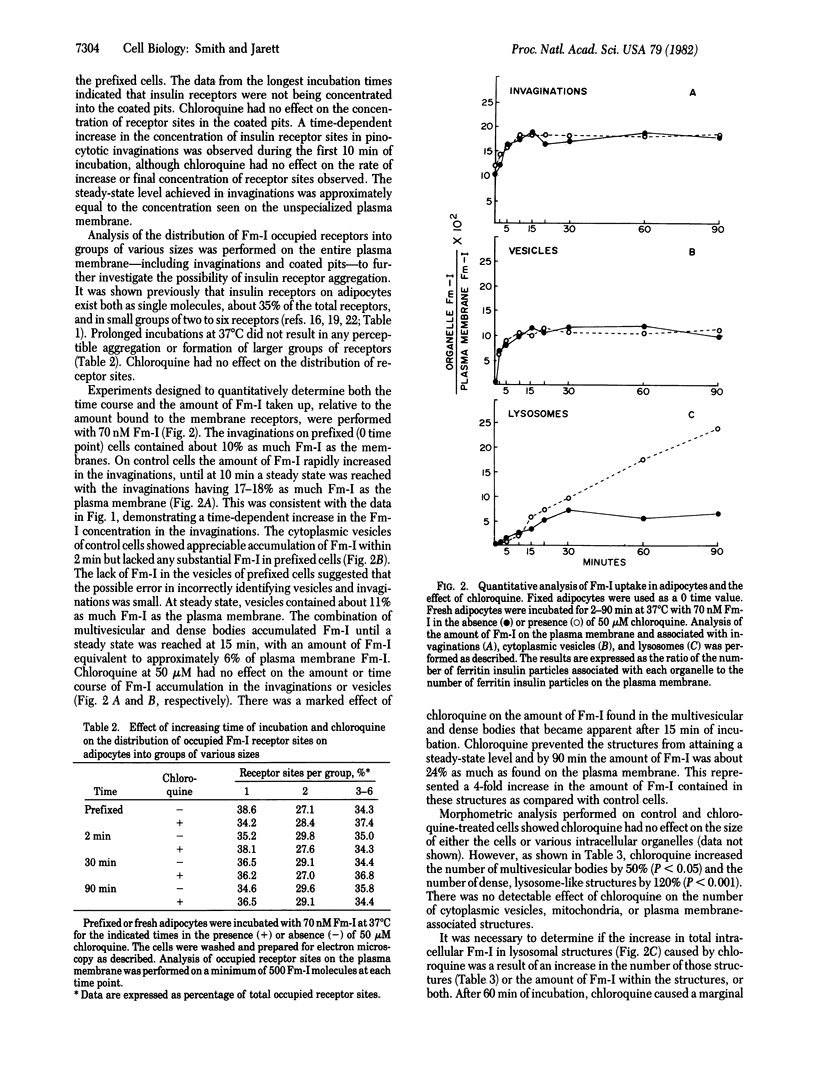
A quantitative morphological analysis of insulin uptake into adipocytes was undertaken to determine the structural basis for chloroquine-induced increases in intracellular insulin. Adipocytes were incubated with ferritin-labeled insulin in the presence or absence of 50 microM chloroquine at 37 degrees C for 2-90 min and the uptake of the hormone conjugate was determined quantitatively. Quantitative morphometry of cellular organelles also was performed. Chloroquine treatment of adipocytes incubated with 70 nM ferritin-labeled insulin resulted in: (i) a 120% increase in the number of lysosomes in the cytoplasm; (ii) a 75% increase in the average concentration of ferritin-labeled insulin in a lysosome; and (iii) a 25% increase in the percentage of lysosomes containing ferritin-labeled insulin. The cumulative result of these effects was a substantial increase in the amount of intact intracellular hormone within the lysosomes. These morphological data are consistent with biochemical data concerning chloroquine-induced accumulation of 125I-labeled insulin in adipocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin D., Jr, Prince M., Marshall S., Davies P., Olefsky J. M. Regulation of insulin receptors: evidence for involvement of an endocytotic internalization pathway. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5975–5978. doi: 10.1073/pnas.77.10.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzone P., Carpentier J. L., Gorden P., Van Obberghen E., Orci L. Polar redistribution of 125I-labelled insulin on the plasma membrane of cultured human lymphocytes. Nature. 1980 Jul 24;286(5771):401–403. doi: 10.1038/286401a0. [DOI] [PubMed] [Google Scholar]
- Berhanu P., Olefsky J. M., Tsai P., Thamm P., Saunders D., Brandenburg D. Internalization and molecular processing of insulin receptors in isolated rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4069–4073. doi: 10.1073/pnas.79.13.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Mulder-Stapel A. A., Daems W. T., Ginsel L. A. The effect of chloroquine on the intralysosomal degradation of cell-coat glycoproteins in the absorptive cells of cultured human small-intestinal tissue as shown by silver proteinate staining. Histochemistry. 1981 Dec;73(3):429–438. doi: 10.1007/BF00495657. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N., Pearse B. M. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon N. D., Smith R. M., Jarett L. Computer assisted analysis of ferritin-insulin receptor sites on adipocytes and the effects of cytochalasin B on groups of insulin receptor sites. J Membr Biol. 1981 Feb 15;58(2):155–160. doi: 10.1007/BF01870977. [DOI] [PubMed] [Google Scholar]
- Goldstein B. J., Livingston J. N. An evaluation of the importance of lysosomal and neutral cytosol proteases in insulin degradation by adipocytes. Endocrinology. 1981 Mar;108(3):953–961. doi: 10.1210/endo-108-3-953. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A., Olefsky J. M. Evidence for insulin-induced internalization and degradation of insulin receptors in rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):427–431. doi: 10.1073/pnas.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammons G. T., Jarett L. Lysosomal degradation of receptor-bound 125I-labeled insulin by rat adipocytes: its characterization and dissociation from the short-term biologic effects of insulin. Diabetes. 1980 Jun;29(6):475–486. doi: 10.2337/diab.29.6.475. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Manipulations of the phagosome-lysosome fusion response in cultured macrophages. Enhancement of fusion by chloroquine and other amines. Exp Cell Res. 1978 Jul;114(2):486–490. doi: 10.1016/0014-4827(78)90516-5. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Maddux B., Goldfine I. D. Chloroquine stimulation of insulin binding to IM-9 lymphocytes: evidence for action at a nonlysosomal site. Biochem Biophys Res Commun. 1981 Dec 15;103(3):863–871. doi: 10.1016/0006-291x(81)90890-1. [DOI] [PubMed] [Google Scholar]
- Jarett L., Schweitzer J. B., Smith R. M. Insulin receptors: differences in structural organization on adipocyte and liver plasma membranes. Science. 1980 Dec 5;210(4474):1127–1128. doi: 10.1126/science.7003710. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Effect of cytochalasin B and D on groups of insulin receptors and on insulin action in rat adipocytes. Possible evidence for a structural relationship of the insulin receptor to the glucose transport system. J Clin Invest. 1979 Apr;63(4):571–579. doi: 10.1172/JCI109338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Electron microscopic demonstration of insulin receptors on adipocyte plasma membranes utilizing a ferritin-insulin conjugate. J Biol Chem. 1974 Nov 10;249(21):7024–7031. [PubMed] [Google Scholar]
- Jarett L., Smith R. M. The natural occurrence of insulin receptors in groups on adipocyte plasma membranes as demonstrated with monomeric ferritin-insulin. J Supramol Struct. 1977;6(1):45–59. doi: 10.1002/jss.400060104. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Ultrastructural localization of insulin receptors on adipocytes. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3526–3530. doi: 10.1073/pnas.72.9.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. The fate of insulin bound to adipocytes. Evidence for compartmentalization and processing. J Biol Chem. 1978 Jul 25;253(14):4900–4906. [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Iwasaki M., Shigeta Y. Receptor mediated insulin degradation decreased by chloroquine in isolated rat adipocytes. J Biochem. 1980 Jul;88(1):39–44. [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Sarver J. A., Vega F. V., Pointer R. H. Actions of insulin in fat cells. Effects of low temperature, uncouplers of oxidative phosphorylation, and respiratory inhibitors. J Biol Chem. 1977 Apr 10;252(7):2226–2233. [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of lysosomotropic agents on insulin interactions with adipocytes. Evidence for a lysosomal pathway for insulin processing and degradation. J Biol Chem. 1979 Oct 25;254(20):10153–10160. [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. The endocytotic-internalization pathway of insulin metabolism: relationship to insulin degradation and activation of glucose transport. Endocrinology. 1980 Dec;107(6):1937–1945. doi: 10.1210/endo-107-6-1937. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna J. A., Haigler H. T., Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego J. L., Chung C. H. Effects of some antimalarials and related substances on intralysosomal proteolysis. Biochem Pharmacol. 1979;28(4):465–470. doi: 10.1016/0006-2952(79)90237-5. [DOI] [PubMed] [Google Scholar]
- Nelson D. M., Smith R. M., Jarett L. Nonuniform distribution and grouping of insulin receptors on the surface of human placental syncytial trophoblast. Diabetes. 1978 May;27(5):530–538. [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Cytochemical contributions to differentiating GERL from the Golgi apparatus. Histochem J. 1977 Sep;9(5):525–551. doi: 10.1007/BF01002901. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M., Chang H. Interactions between insulin and its receptors after the initial binding event. Functional heterogeneity and relationships to insulin degradation. Diabetes. 1979 May;28(5):460–471. doi: 10.2337/diab.28.5.460. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Patel B. A., Khan M. N., Bergeron J. J. Effect of chloroquine on the internalization of 125I-insulin into subcellular fractions of rat liver. Evidence for an effect of chloroquine on Golgi elements. J Biol Chem. 1982 May 25;257(10):5789–5799. [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. A simplified method of producing biologically active monomeric ferritin-insulin for use as a high resolution ultrastructural marker for occupied insulin receptors. J Histochem Cytochem. 1982 Jul;30(7):650–656. doi: 10.1177/30.7.7050238. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Internalization and degradation of fat cell-bound insulin. Separation and partial characterization of subcellular vesicles associated with iodoinsulin. J Biol Chem. 1979 Oct 10;254(19):9786–9794. [PubMed] [Google Scholar]
- WILLIAMSON J. R. ADIPOSE TISSUE. MORPHOLOGICAL CHANGES ASSOCIATED WITH LIPID MOBILIZATION. J Cell Biol. 1964 Jan;20:57–74. doi: 10.1083/jcb.20.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Maxfield F. R., Pastan I. H. alpha 2 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated regions. J Cell Biol. 1979 Sep;82(3):614–625. doi: 10.1083/jcb.82.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner-Gebhart A. M., Brabec R. K., Gray R. H. Morphometric studies of chloroquine-induced changes in hepatocytic organelles in the rat. Exp Mol Pathol. 1980 Oct;33(2):144–152. doi: 10.1016/0014-4800(80)90015-5. [DOI] [PubMed] [Google Scholar]


