Figure 1. ORAI1, STIM1 and STIM2 mediate SOCE and control gene expression in distinct T cell subsets.
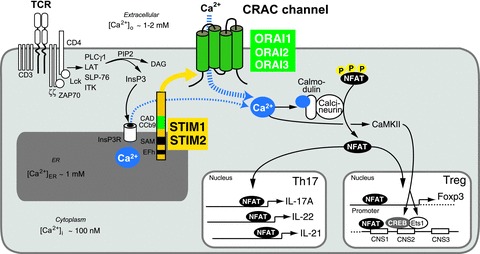
A, SOCE is activated following T cell receptor (TCR) or B cell receptor (BCR, not shown) ligation in T and B cells. In T cells, the protein tyrosine kinases Lck and ZAP-70 initiate a signalling cascade that results in the activation of PLCγ1 and production of InsP3, a second messenger whose binding to the InsP3 receptor leads to the release of Ca2+ from the ER. The subsequent reduction of [Ca2+]ER causes the dissociation of Ca2+ from EF hand (EFh) domains in the N terminus of STIM1 and STIM2, unfolding of the adjacent EFh-SAM domains and oligomerization of STIM molecules. Oligomerized STIM assembles in puncta localized at junctions formed by the ER and plasma membrane to which ORAI channels are recruited. STIM1 binds to and activates ORAI1 via a CRAC activation domain (CAD or CCb9) in its C terminus. Opening of ORAI CRAC channels in the plasma membrane results in sustained Ca2+ influx and activation of several Ca2+ regulated enzymes and transcription factors. Of particular importance in lymphocytes is the serine/threonine phosphatase calcineurin, which dephosphorylates NFAT (nuclear factor of activated T cells) and thereby enables it to translocate to the nucleus and bind to promoters and conserved non-coding DNA sequences (CNS) of many target genes. The expression of several cytokines (IL-17A, IL-22, IL-21) in Th17 cells and the lineage-specific transcription factor Foxp3 in Treg cells are regulated by NFAT. (Note: only NFAT and other Ca2+ dependent transcription factors regulating cytokine and Foxp3 expression are shown in this figure.)
