Figure 8. Proposed model for modulation of L-type Ca2+ channel by oxygen.
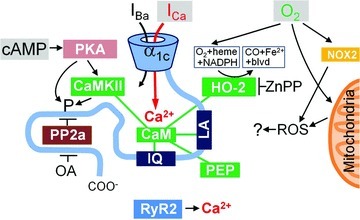
The carboxy-tail of the α1C subunit includes LA and IQ motifs for calmodulin- (CaM) mediated interactions and a binding site for protein phosphatase 2a (PP2a). CaM is shown as also interacting with CaMKII, haem oxygenase-2 (HO-2) and inhibitory peptide290−309 (PEP). The primary inputs to the system are thought to be: cAMP leading to PKA-mediated phosphorylation (left), permeation of Ca2+ through the channel to a restricted space (middle), and molecular oxygen (O2) that binds to haem/HO. PKA may act directly or via CaMKII and may have multiple phoshporylation targets (including HO-2). Similarly okadaic acid (OA) may promote phosphorylation inhibiting different phosphatases. ROS generation by mitochondria or membrane bound NOX proteins, CO-mediated signalling and ICa-triggered release of Ca2+ from the SR are thought to be of little or no importance for the rapid suppression of the Ca2+ channel at the onset of hypoxia.
