Abstract
Objective
Moraxella catarrhalis is an important pathogen in the exacerbation of chronic obstructive pulmonary disease. The aim of this study was to assess the clinical and pulmonary thin-section CT findings in patients with acute M. catarrhalis pulmonary infection.
Methods
Thin-section CT scans obtained between January 2004 and March 2009 from 292 patients with acute M. catarrhalis pulmonary infection were retrospectively evaluated. Clinical and pulmonary CT findings in the patients were assessed. Patients with concurrent infection including Streptococcus pneumoniae (n = 72), Haemophilus influenzae (n = 61) or multiple pathogens were excluded from this study.
Results
The study group comprised 109 patients (66 male, 43 female; age range 28–102 years; mean age 74.9 years). Among the 109 patients, 34 had community-acquired and 75 had nosocomial infections. Underlying diseases included pulmonary emphysema (n = 74), cardiovascular disease (n = 44) or malignant disease (n = 41). Abnormal findings were seen on CT scans in all patients and included ground-glass opacity (n = 99), bronchial wall thickening (n = 85) and centrilobular nodules (n = 79). These abnormalities were predominantly seen in the peripheral lung parenchyma (n = 99). Pleural effusion was found in eight patients. No patients had mediastinal and/or hilar lymph node enlargement.
Conclusions
M. catarrhalis pulmonary infection was observed in elderly patients, often in combination with pulmonary emphysema. CT manifestations of infection were mainly ground-glass opacity, bronchial wall thickening and centilobular nodules.
Moraxella catarrhalis is a Gram-negative, aerobic, oxidase-positive diplococcus that was first described in 1896 [1]. The pathogen, also known as Micrococcus catarrhalis, Neisseria catarrhalis and Brahamella catarrhalis, is a clinically important pathogen and is a common cause of respiratory infections, particularly otitis media in children and lower respiratory tract infection in elderly patients [2-5]. M. catarrhalis is considered to be the third most common and most important cause of bronchopulmonary infections after Streptococcus pneumoniae and Haemophilus influenzae [6,7]. In the Alexander project in Europe and the US between 1992 and 1993, M. catarrhalis was identified in 13.5% of bacterial isolates [8].
M. catarrhalis has also gained attention as a nosocomial respiratory pathogen and as a community-acquired pathogen. On the basis of epidemiological evidence, the spread of M. catarrhalis was suggested to occur within the hospital environment [9,10]. McLeod et al [11] reported that 43 of 81 patients (53%) with M. catarrhalis infection were infected in a hospital and that the infection was associated with the proximity of the patient to other patients. Most nosocomial infections with M. catarrhalis involve the respiratory tract and outbreaks have been reported in respiratory units and paediatric intensive care units [10,12].
M. catarrhalis infection has received increasing attention because it is an important factor in the acute exacerbation of chronic obstructive pulmonary disease (COPD). Acute exacerbation is a frequent event during the prolonged chronic course of COPD, which entails significant morbidity and mortality. The main aetiology for the majority of episodes is infection.
Al-Anazi et al [13] reported a CT image of pneumonia associated with M. catarrhalis in a haematopoietic stem cell transplant patient. However, to the best of our knowledge, no other English-language studies of pulmonary CT findings in patients with acute M. catarrhalis pulmonary infection have been published. Therefore, this study aimed to assess the clinical and pulmonary thin-section CT findings in acute M. catarrhalis pulmonary infection.
Methods and materials
Our institutional review board approved this retrospective study and waived informed consent.
We retrospectively identified 292 patients with acute M. catarrhalis pulmonary infection who had undergone pulmonary thin-section CT scans between January 2004 and March 2009 at four institutions. We excluded 72 patients infected with S. pneumoniae, 61 with H. influenzae, 37 with Staphylococcus aureus, 25 with meticillin-resistant Staphylococcus aureus (MRSA), 20 with Pseudomonas aeruginosa and some with other pathogens, who were diagnosed with concurrent infectious diseases by serological tests and clinical findings. Of the patients with concurrent infection, 44 were infected with more than one organism. Seven patients with pulmonary oedema, five with recurrence of malignancy and one with pulmonary haemorrhage were also excluded. Moreover, four cases with acute M. catarrhalis pulmonary infection were excluded because of poor image quality caused by motion artefacts, inadequate window level settings or for which hard copies of the CT film had been destroyed. Thus, the study group comprised 109 patients (66 male, 43 female; age range 28–102 years; mean age 74.9 years) with acute M. catarrhalis pulmonary infection. No patients with human immunodeficiency virus (HIV) infection or smoking-related diseases such as desquamative interstitial pneumonia or Langerhans cell histiocytosis were included in this study.
The diagnosis was established by isolation of M. catarrhalis from sputum in 100 patients, sputum from the trachea in 7 and bronchoalveolar lavage fluid in 2. A patient was considered to have community-acquired pneumonia if, at the time of hospital admission, he/she presented with cough (with or without sputum), fever, leukocytosis or leukopenia and had pulmonary infiltrates on chest radiographs. No patient had been admitted to or treated in a hospital 2 weeks prior to admission. Nosocomial pneumonia was defined as pneumonia that occurred 48 h or more after admission, which was not incubated at the time of admission [14]. Among the 109 patients, 34 had community-acquired and 75 had nosocomial infections.
The study group included 74 patients with pulmonary emphysema. In addition, patients with cardiovascular disease (n = 44), post-operative status for malignant disease (n = 41), diabetes mellitus (n = 18) or liver disorders (n = 16) were included in the study (Table 1).
Table 1. Patient characteristics and underlying conditions.
| Characteristic/condition | No. of patients (%) |
| Sex (male/female) | 66/43 |
| Pulmonary emphysema | 74 (67.9) |
| Smoking habit | 47 (43.1) |
| Cardiac disease | 44 (40.4) |
| Alcoholic | 25 (22.9) |
| Diabetes mellitus | 18 (16.5) |
| Liver disorder | 16 (14.7) |
| Collagen disease | 6 (5.5) |
| Renal failure | 4 (3.7) |
| Malignancy | 41 (37.6) |
| lung cancer | 15 (13.8) |
| gastric cancer | 14 (12.8) |
| oesophageal cancer | 10 (9.2) |
| colon cancer | 2 (1.8) |
| Presenting symptoms | |
| cough | 103 (94.5) |
| sputum | 81 (74.3) |
| fever | 73 (67.0) |
| dyspnea | 19 (17.4) |
| general weakness | 18 (16.5) |
An alcoholic was defined as an individual with an alcohol consumption of ≥80 g day–1 during the past 2 years [15]; a patient was considered to be a heavy smoker if he/she had smoked more than 10 pack–years. Overall, 25 patients were alcoholic, 47 were chronic smokers and 12 were both alcoholic and chronic smokers (Table 1).
CT
Examinations
Thin-section CT examinations were performed at 4 institutions using a variety of scanners with 1-mm collimation (n = 14) at 10-mm intervals from the apex of the lung to the diaphragm or volumetrically with a multidetector CT scanner (n = 95) with 1-mm reconstruction. The scans were obtained with the patient in the supine position at full inspiration and images were reconstructed using a high spatial frequency algorithm.
Images were captured at window settings that allowed viewing of the lung parenchyma (window level −600 to −700 HU; window width 1200–1500 HU) and the mediastinum (window level 20–40 HU; window width 400 HU).
The pulmonary CT scan was performed within 1–6 days (mean 4.7 days) after the onset of respiratory symptoms. Intravenously administered contrast material was used in eight patients.
Image interpretation
Two chest radiologists (with 21 and 13 years of experience in chest CT image interpretation), who were aware of the underlying diagnoses, retrospectively and independently interpreted all CT images on workstations. Conclusions were reached by consensus. An average of two sessions per week was reserved to review the CT scans; about 50 sessions were carried out in total.
CT images were assessed for several radiological features: ground-glass opacity, consolidation, nodules, centrilobular nodules, bronchial wall thickening, interlobular septal thickening, intralobular reticular opacity, bronchiectasis, enlarged hilar/mediastinal lymph node(s) (>1 cm diameter short axis) and pleural effusion. Areas of ground-glass opacity were defined as hazy increases in opacity without obscured vascular markings [16,17]. Areas of consolidation were defined as areas of increased opacity that obscured normal lung markings [16,17]. Centrilobular nodules were defined as those present around the peripheral pulmonary arterial branches or 3–5 mm from the pleura, interlobular septa or pulmonary veins. Interlobular septal thickening was defined as abnormal widening of interlobular septa [17]. Intralobular reticular opacity was considered present when interlacing line shadows were separated by a few millimetres [16,17].
The distribution of parenchymal disease was also noted. We also assessed whether the abnormal findings were located unilaterally or bilaterally. If the main lesion was predominantly located in the inner third of the lung, the disease was classified as having a central distribution. By contrast, if the lesion was predominantly located in the outer third of the lung, the disease was classified as having a peripheral distribution. If the lesions showed no predominant distribution, the disease was classified as having a random distribution. In addition, zonal predominance was classified as upper, lower or random. Upper lung zone predominance was defined as most abnormalities being seen at a level above the tracheal carina, whereas lower zone predominance was defined as most abnormalities being below the upper zone. When abnormalities showed no definite zonal predominance, the lung disease was considered to have a random distribution.
Results
Clinical features
The patient characteristics and underlying conditions are summarised in Table 1. All patients had respiratory symptoms and most showed rapid progression of their respiratory symptoms. The most common presenting symptoms were cough (103 patients, 94.5%), followed by sputum (81 patients, 74.3%), fever (73 patients, 67.0%) and dyspnoea (19 patients, 17.4%).
CT patterns
Chest CT scans revealed abnormalities in all patients with M. catarrhalis pneumonia (Table 2). Among the 109 patients, ground-glass opacity (n = 99, 90.8%) (Figures 1–4) was the most frequently observed abnormality, followed by bronchial wall thickening (n = 85, 78.0%) (Figures 1–4), centrilobular nodules (n = 79, 72.5%) (Figures 1 and 3), consolidation (n = 53, 48.6%) (Figures 1 and 2) and bronchiectasis (n = 42, 38.5%) (Figure 3). Intralobular reticular opacity (n = 25, 22.9%) and interlobular septal thickening (n = 10, 9.2%) were also observed. The most frequently observed combination was ground-glass opacity and bronchial wall thickening (n = 80, 73.4%) (Figures 1–4), followed by ground-glass opacity and centrilobular nodules (n = 71, 65.1%) (Figures 1 and 3) and bronchial wall thickening and centrilobular nodules (n = 69, 63.3%) (Figures 1 and 3).
Table 2. Thoracic CT findings in 109 patients.
| Finding | No. of patients (%) |
| Ground-glass opacity | 99 (90.8) |
| Bronchial wall thickening | 85 (78.0) |
| Centrilobular nodules | 79 (72.5) |
| Consolidation | 53 (48.6) |
| Bronchiectasis | 42 (38.5) |
| Intralobular reticular opacity | 25 (22.9) |
| Interlobular septal thickening | 10 (9.2) |
| Nodules | 3 (2.8) |
| Pleural effusion | 8 (7.3) |
| Lymph node enlargement | 0 (0) |
Figure 1.
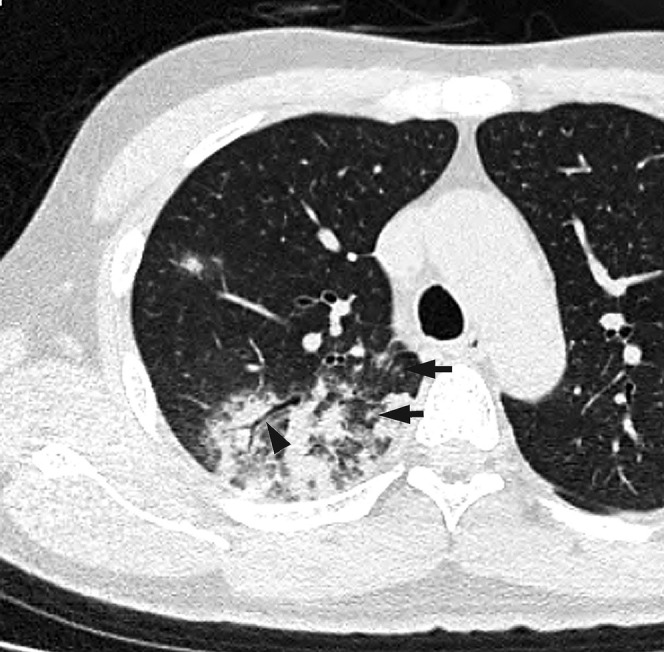
Acute Moraxella catarrhalis infection in a 42-year-old alcoholic male with diabetes mellitus, 2 days after onset of fever and cough with sputum. A transverse thin-section CT of the right upper lobe shows consolidation, ground-glass opacity, bronchial wall thickening (arrowhead) and centrilobular nodules (arrow).
Figure 4.
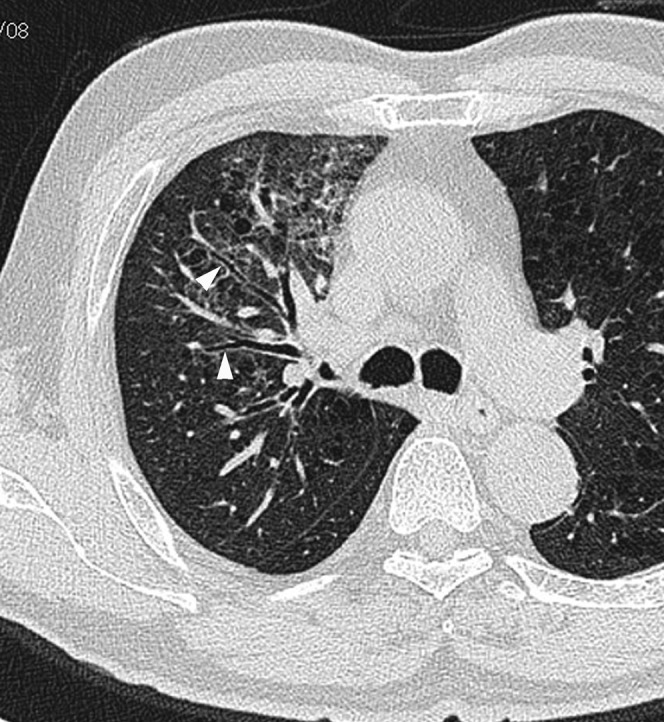
Acute Moraxella catarrhalis infection in a 76-year-old alcoholic male with pulmonary emphysema, 3 days after onset of fever and cough. A transverse thin-section CT at the tracheal carina level shows ground-glass opacity and bronchial wall thickening (arrowhead).
Figure 2.
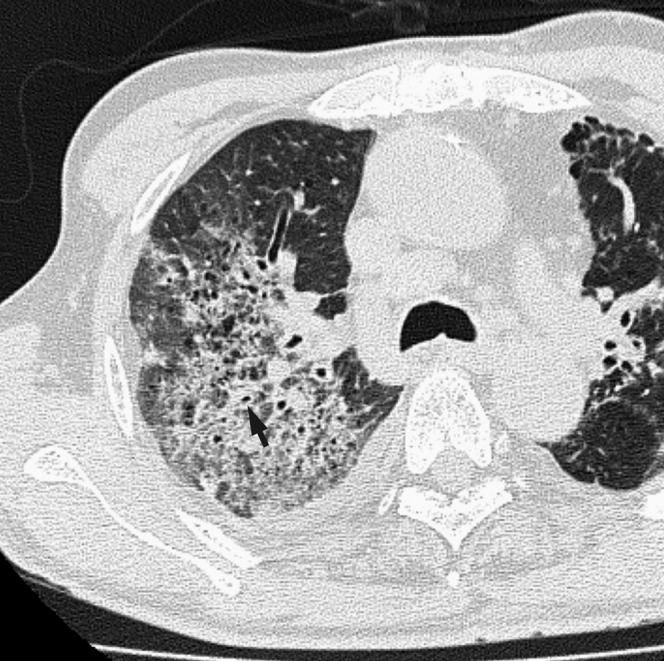
Acute Moraxella catarrhalis infection in a 75-year-old alcoholic male with pulmonary emphysema, 4 days after onset of fever, cough and dyspnea. A transverse thin-section CT of the right upper lobe shows consolidation, ground-glass opacity and bronchial wall thickening (arrow). Pleural effusion is also present.
Figure 3.
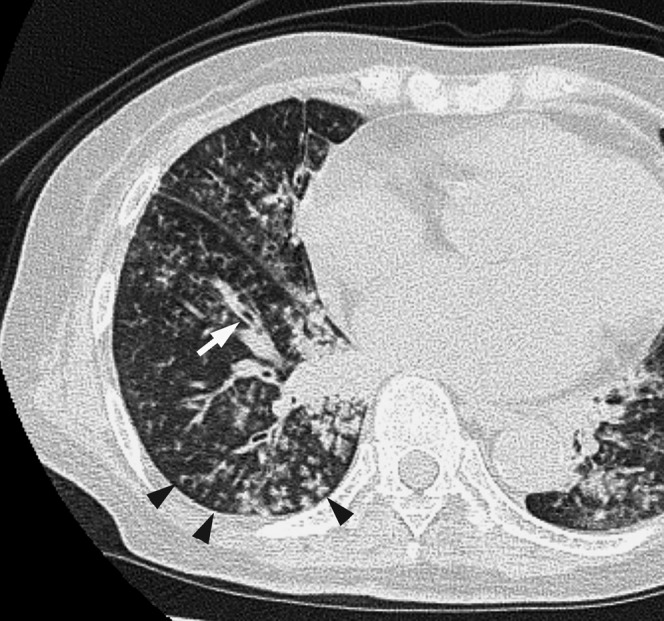
Acute Moraxella catarrhalis infection in a 72-year-old alcoholic female with cardiovascular disease and renal failure, 3 days after the onset of fever and cough with sputum. A transverse thin-section CT of the right lower lobe shows centrilobular nodules (arrowheads), bronchial wall thickening (arrow) and mild bronchiectasis.
Disease distribution
Of the 109 patients with M. catarrhalis pneumonia, abnormal findings were found bilaterally in 63 patients (57.8%), unilaterally in 46 patients (42.2%) and in the periphery in 99 patients (90.8%) (Figures 1, 2 and 4). Ten patients showed a random distribution (9.2%) (Figure 3), no patients had a predominantly central distribution. The predominant zonal distribution was the upper zone in 21 patients (19.3%) (Figures 1, 2 and 4), lower zone in 60 patients (55.0%) (Figure 3) and of random distribution in 28 patients (25.7%).
Effusion and lymph nodes
Bilateral pleural effusions were found in two patients (1.8%) and unilateral pleural effusion was found in six patients (5.5%) (Figure 2) with acute M. catarrhalis infection. No patient had mediastinal and/or hilar lymph node enlargement.
Follow-up study
All 109 patients underwent antibiotic therapy. In 32 of 34 patients with community-acquired infections (94.1%), the abnormal findings improved on follow-up CT examinations or chest radiographs. In the remaining two patients (5.9%) with pulmonary emphysema and cardiac disease, however, abnormal findings such as ground-glass opacity and consolidation on follow-up CT worsened and the patients died. By comparison, in 70 of 75 patients with nosocomial infections (93.3%), the abnormal findings improved on follow-up CT or radiographs. In the remaining five patients (6.7%), which included four with pulmonary emphysema and one with cardiovascular disease and diabetes mellitus, the abnormal parenchymal findings and pleural effusions worsened and the patients died.
Discussion
M. catarrhalis is one of the most clinically important Gram-negative bacterial pathogens and is of great concern worldwide for several reasons: infections can exacerbate COPD, they can cause pneumonia (particularly in older adults) and M. catarrhalis is a nosocomial respiratory tract pathogen [18].
Exacerbation of COPD can be caused by many factors, including environmental irritants, heart failure or non-compliance with medication use [19]. However, most exacerbations result from bacterial or viral infections [20]. Bacterial infection is a factor in 70–75% of exacerbations; up to 60% are caused by S. pneumoniae, H. influenzae or M. catarrhalis [21].
The majority of respiratory isolates containing M. catarrhalis are from elderly patients [2,22-24]. Wright et al [2] analysed the respiratory isolates obtained at one hospital in Texas and found that 81% of patients with M. catarrhalis infection were aged over 55 years. The authors also noted a high short-term mortality rate in elderly patients: 45% of patients died within 3 months of acquiring M. catarrhalis pneumonia. Most elderly patients who experience pneumonia as a result of M. catarrhalis infection have underlying cardiopulmonary diseases, including COPD, bronchiectasis, congestive heart failure or predisposition to aspiration. Other predisposing conditions associated with M. catarrhalis infection include corticosteroid therapy, diabetes mellitus and malignancies [22,23,25-28]. Factors contributing to the high incidence of respiratory infections in this age group include immunosuppression and enhanced adherence of M. catarrhalis to epithelial cells in elderly patients [29,30].
Moreover, many nosocomial outbreaks of M. catarrhalis infections have been reported. Most of these outbreaks involved respiratory tract infections and some occurred exclusively in pulmonary units [10,12,31].
The average age of patients in our study with M. catarrhalis infection alone was 74.9 years, which is similar to that in previous reports [2,22-24]. S. pneumoniae, Klebsiella pneumoniae, Mycoplasma pneumoniae and Chlamydia pneumoniae are also common pathogens involved in community-acquired or nosocomial pneumonia. The average age of patients with M. catarrhalis infection tended to be greater than that of patients with pneumonia caused by other pathogens such as S. pneumoniae, K. pneumoniae, M. pneumoniae or C. pneumoniae (60 years, 61.5 years, 47.3 years and 57.7 years, respectively) [32-34]. Among the 109 patients with pulmonary infection caused by M. catarrhalis alone, pulmonary emphysema (67.9%) was the most commonly associated condition, followed by smokers (43.1%), cardiovascular disease (40.4%), malignant disease (37.6%), alcoholism (22.9%) and diabetes mellitus (16.5%). Underlying diseases such as pulmonary emphysema, cardiovascular disease or malignancy were more frequently seen in patients with M. catarrhalis than in patients with K. pneumoniae pneumonia alone (67.9% vs 17.7%, 40.4% vs 19.7% and 37.6% vs 18.2%, respectively) [33].
Among the 109 patients in this study, 34 had community-acquired and 75 had nosocomial infections; this observation is similar to that presented in an earlier report [11] and suggests that M. catarrhalis is a nosocomial respiratory pathogen and a community-acquired pathogen.
Regarding the presenting symptoms, all patients in the present study had several complaints such as fever, cough and sputum. There were no significant differences between patients with other pneumonias such as K. pneumoniae, M. pneumoniae or C. pneumoniae [33,34].
In the present study, the mortality rate was 6.4% (7 of 109 patients), which was lower than in previous reports [2,35]. However, this might be because most of the earlier studies were published in the pre-antibiotic era or in an era of minimal antibiotic use. In addition, no previous studies have evaluated patients with M. catarrhalis infection in the absence of any other pathogens. In the present study, one or more additional pathogens, such as S. pneumoniae, H. influenzae, S. aureus or MRSA, were identified in 183 of 292 patients (62.7%) with acute M. catarrhalis pulmonary infection; patients diagnosed with concurrent infectious diseases were excluded from this study. Therefore, the mortality rates in our patients with M. catarrhalis pulmonary infection might have been lower than those found in previous studies.
There are several case reports of patients with M. catarrhalis pulmonary infection [13,35-37]; however, few included chest radiographs. Cheepsattayakorn et al [35] reported M. catarrhalis pneumonia in acquired immunodeficiency syndrome and reported that the chest radiographs showed patchy infiltration in both lower lobes with minimal pleural effusion. Al-Anazi et al [13] presented two cases of M. catarrhalis pneumonia in haematopoietic stem cell transplant patients. The chest radiograph of a 17-year-old female with acute myeloid leukaemia showed bilateral pulmonary infiltrates, which were more prominent on the left; the chest CT image showed nodular infiltration involving the left lower lobe and the lateral segment of the right lower lobe. In the other patient, a 50-year-old male with acute myeloid leukaemia, the chest radiograph showed bronchopneumonia. To the best of our knowledge, however, no other English-language studies of pulmonary CT findings in patients with acute M. catarrhalis pulmonary infection have been published.
We retrospectively evaluated the CT findings of 109 patients with acute M. catarrhalis pulmonary infection. The most common CT findings were ground-glass opacity followed by bronchial wall thickening, centrilobular nodules, consolidation and bronchiectasis. The abnormal findings were predominantly seen in the lower zone and in the peripheral lungs.
Nambu et al [32] reported that the CT findings in 41 patients with S. pneumoniae pneumonia consisted mainly of consolidation, reticular opacity and centrilobular nodules (90%, 39% and 32%, respectively). Previously, we have reported chest CT findings in 198 patients with acute K. pneumoniae pneumonia alone [33], in 42 patients with M. pneumoniae pneumonia alone [34] and in 40 patients with C. pneumoniae pneumonia alone [34]. The frequency of bronchial wall thickening with M. catarrhalis infection was higher than that with S. pneumoniae, K. pneumoniae or C. pneumoniae infection (78.0% vs 41%, 26.3% and 35.0%, respectively). Moreover, the frequency of centrilobular nodules with M. catarrhalis infection was also higher than that with S. pneumoniae, K. pneumoniae or C. pneumoniae infection (72.5% vs 56%, 4.0% and 7.5%, respectively). The frequencies of these features with M. catarrhalis infection were relatively similar to those with M. pneumoniae infection; however, the average age of patients with M. catarrhalis infection was significantly higher than for those with M. pneumoniae (74.9 years vs 47.3 years). The frequency of consolidation in patients infected with M. catarrhalis was lower than for those with S. pneumoniae or K. pneumoniae infection (48.6% vs 90% and 91.4%, respectively). In addition, intralobular reticular opacity was less frequently seen with M. catarrhalis infection than with K. pneumoniae or C. pneumoniae (22.9% vs 85.9% and 70.0%, respectively).
Bilateral pleural effusion was seen in two patients (1.8%) and unilateral pleural effusion in six patients (5.5%) with M. catarrhalis infection. The frequency of pleural effusions was lower than in patients with other pathogens such as S. pneumoniae, C. pneumoniae, M. pneumoniae or K. pneumoniae (20%, 25–30%, 9.5–20% and 53%, respectively) [32-34].
No patient in our present study had mediastinal and/or hilar lymph node enlargement. The frequency of lymph node enlargement was also lower than in patients with S. pneumoniae, C. pneumoniae or M. pneumoniae (36%, 5–33% and 7.1–10%, respectively) [32-34].
It should be noted that there are several limitations to our study. Firstly, this was a retrospective study and CT images were interpreted by consensus. Secondly, the thin-section CT images were obtained at several institutions using different protocols.
In summary, M. catarrhalis pulmonary infection was observed in elderly patients, often in combination with pulmonary emphysema. The CT manifestations in patients with M. catarrhalis pulmonary infection consisted mainly of ground-glass opacity, bronchial wall thickening and centrilobular nodules in the lung periphery; there was a low frequency of pleural effusion or lymph node enlargement.
References
- 1.Frosch P, Kolle W. Die Mikrokokken Flugge C, ed. Die Mikroorganismen. Leipzig: Verlag von Vogel, 1956;154–5 [Google Scholar]
- 2.Wright PW, Wallace RJ, Shepherd JR. A descriptive study of 42 cases of Branhamella catarrhalis pneumonia. Am J Med 1990;88:2S–8S [DOI] [PubMed] [Google Scholar]
- 3.West M, Berk SL, Smith JK. Branhamella catarrhalis pneumonia. Southern Med J 1982;75:1021–3 [DOI] [PubMed] [Google Scholar]
- 4.Wallace RJ, Musher DM. In honor of Dr. Sara Braham, a star is born. The realization of Brahamella catarrhalis as a respiratory pathogen. Chest 1986;90:447–50 [DOI] [PubMed] [Google Scholar]
- 5.Kovatch AL, Wald ER, Michaels RH. Beta-lactamase producing Brahamella catarrhalis causing otitis media in children. J Pediatrics 1983;102:261–4 [DOI] [PubMed] [Google Scholar]
- 6.Volkov IK, Katosova LK, Scherbakova Nlu, Kliukina LP. Moraxella catarrhalis in chronic and relapsing respiratory tract infections in children. Antibiot Khimioter 2004;49:43–7 [PubMed] [Google Scholar]
- 7.Enright MC, McKenzie H. Moraxella (Branhamella) catarrhalis: clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol 1997;46:360–71 [DOI] [PubMed] [Google Scholar]
- 8.Felmingham D, Gruneberg RN. A multicentre collaborative study of the antimicrobial susceptibility of community-acquired, lower respiratory tract pathogens 1992–1993: The Alexander Project. J Antimicrob Chemother 1996;38(Suppl A):1–57 [DOI] [PubMed] [Google Scholar]
- 9.Calder MA, Croughan MJ, McLeod DT, Ahmad F. The incidence and antibiotic susceptibility of Branhamella catarrhalis in respiratory infections. Drugs 1986;31(Suppl 3):11–6 [DOI] [PubMed] [Google Scholar]
- 10.Cook PP, Hecht DW, Snydman DR. Nosocomial Branhamella catarrhalis in a paediatric intensive care unit; risk factors for disease. J Hosp Infect 1989;13:299–307 [DOI] [PubMed] [Google Scholar]
- 11.McLeod DT, Ahmad F, Power JT, Calder MA, Seaton A. Bronchopulmonary infection due to Branhamella catarrhalis. Br Med J 1983;287:1446–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards SJ, Greening AP, Enright MC, Morgan MG, McKenzie H. Outbreak of Branhamella catarrhalis in a respiratory unit. Thorax 1993;48:91–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Anazi KA, Al-Fraih FA, Chaudhri NA, Al-Mohareb FI. Pneumonia caused by Moraxella catarrhalis in haematopoietic stem cell transplant patients. Report of two cases and review of the literature. Libyan J Med 1997;23:144–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Thoracic Society; Infectious Disease Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416 [DOI] [PubMed] [Google Scholar]
- 15.Torres A, Serra-Batlles J, Ferrer A, Jimenez P, Celis R, Rodriguez-Rooson R. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312–8 [DOI] [PubMed] [Google Scholar]
- 16.Webb WR, Muller NL, Naidich DP. High-resolution computed tomography findings of lung disease. In: High-resolution CT of the lung. 3rd edn Philadelphia, PA: Lippincott Williams & Wilkins, 2001:71–192 [Google Scholar]
- 17.Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327–31 [DOI] [PubMed] [Google Scholar]
- 18.Murphy TF. Lung infections. Branhamella catarrhalis: epidemiological and clinical aspects of a human respiratory tract pathogen. Thorax 1998;53:124–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voelkel NF, Tuder R. COPD: exacerbation. Chest 2000;117(5 Suppl 2):376S–9S [DOI] [PubMed] [Google Scholar]
- 20.Fein A, Fein AM. Management of acute exacerbations in chronic obstructive pulmonary disease. Curr Opin Pul Med 2006;6:122–6 [DOI] [PubMed] [Google Scholar]
- 21.Soler N, Torres A, Ewing S, Gonzalez J, Celis R, El-Ebiary M, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998;157:1498–505 [DOI] [PubMed] [Google Scholar]
- 22.Hager H, Verghese A, Alvarez S, Berk SL. Branhamella catarrhalisis respiratory infections. Rev Infect Dis 1987;9:1140–9 [DOI] [PubMed] [Google Scholar]
- 23.Chin NK, Kumarasinghe G, Lim TK. Moraxella catarrhalis respiratory infection in adults. Singapore Med J 1993;34:409–11 [PubMed] [Google Scholar]
- 24.Boyle FM, Georghiou PR, Tilse MH, McCormack JG. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust 1991;154:592–6 [DOI] [PubMed] [Google Scholar]
- 25.Nicotra B, Rivera M, Luman JI, Wallace RJ. Branhamella catarrhalisis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch Intern Med 1986;146:890–3 [PubMed] [Google Scholar]
- 26.Slevin NJ, Aitken J, Thornley PE. Clinical and microbiological features of Branhamella catarrhalisis bronchopulmonary infections. Lancet 1984;1:782–3 [DOI] [PubMed] [Google Scholar]
- 27.Capewell S, McLeod DT, Croughan MJ, Ahmad F, Calder MA, Seaton A. Pneumonia due to Branhamella catarrhalisis. Thorax 1988;43:929–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barreiro B, Esteban L, Prats E, Verdaguer E, Dorca J, Manresa F. Branhamella catarrhalisis respiratory infections. Eur Respir J 1992;5:675–9 [PubMed] [Google Scholar]
- 29.Catlin B. Branhamella catarrhalisis: an organism gaining respect as a pathogen. Clin Microbiol Rev 1990;3:293–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carr B, Walsh JB, Coakley D, Scott T, Mulvihill E, Keane C. Effect of age on adherence of Branhamella catarrhalisis to buccal epithelial cells. Gerontology 1989;35:127–9 [DOI] [PubMed] [Google Scholar]
- 31.Denamur E, Suermondt G, Debroca A, Defouilloy C, Laurans G, Muir JF, et al. Nosocomial pulmonary infections caused by Branhamella catarrhalisis in intensive care units. Agressologie 1989;30:251–3 [PubMed] [Google Scholar]
- 32.Nambu A, Saito A, Araki T, Ozawa K, Hiejima Y, Akao M, et al. Chlamydia pneumoniae: comparison with findings of Mycoplasma pneumoniae and Streptococcus pneumoniae at thin-section CT. Radiology 2006;238:330–8 [DOI] [PubMed] [Google Scholar]
- 33.Okada F, Ando Y, Honda K, Nakayama T, Kiyonaga M, Ono A, et al. Clinical and pulmonary thin-section CT findings in acute Klebsiella pneumoniae pneumonia. Eur Radiol 2009;19:809–15 [DOI] [PubMed] [Google Scholar]
- 34.Okada F, Ando Y, Wakisaka M, Matsumoto S, Mori H. Chlamydia pneumonia pneumonia and Mycoplasma pneumoniae pneumonia: comparison of clinical findings and CT findings. J Comput Assist Tomogr 2005;29:626–32 [DOI] [PubMed] [Google Scholar]
- 35.Cheepsattayakorn A, Tharavichitakul P, Dettrairat S, Sutachai V. Moraxella catarrhalisis pneumonia in an AIDS patient: a case report. J Med Assoc Thai 2009;92:284–9 [PubMed] [Google Scholar]
- 36.Thorsson B, Haraldsdottir V, Kristjansson M. Moraxella catarrhalisis bacteremia. A report on 3 cases and a review of the literature. Scand J Infect Dis 1998;30:105–9 [DOI] [PubMed] [Google Scholar]
- 37.Ioannidis JP, Worthington M, Griffiths JK, Snydman DR. Spectrum and significance of bacteremia due to Moraxella catarrhalisis. Clin Infect Dis 1995;21:390–7 [DOI] [PubMed] [Google Scholar]


