Abstract
Objectives
Incidental findings (IF) are becoming increasingly common due to the proliferation of imaging research. IFs can be life-changing for “healthy” volunteers. This study examined variation in IF management in UK research studies of healthy volunteers, including comparison with ethical and legal guidelines, thus providing baseline data and informing future practice.
Methods
Questionnaire of participant background [medical/non-medical; radiologist/non-radiologist; years as principal investigator (PI)], type of research (involving children or not), institutional policy, volunteer information, radiologist involvement in reporting scans and IF disclosure mechanisms. Investigator's current and perceived “ideal” practice was examined. Participants were PIs performing imaging research of healthy volunteers approved by UK ethics committees (2006–2009).
Results
63/146 (43%) surveys completed. 54/61 (88.5%) had site-specific guidelines. Information commonly provided to volunteers should IF be found: personal data (51/62; 82%), contingency plans (54/62; 87%) and disclosure to general practitioner (GP)/treating physician (47/62; 76%). PIs used different strategies for image review. Commonest: radiologist reports research scans only when researcher suspicious of IF [15/57 (26%) compared with 5/28 (16%) in ideal practice]. Commonest ideal reporting strategy: routine reporting by specialist radiologists [9/28 (29%) compared with 8/57 (14%) in current practice]. 49/56 (87.5%) have a standardised disclosure contingency plan, usually involving GP. PIs most commonly disclosed IFs to volunteers when judged relevant (27/58; 47%), most commonly face to face (22/54; 41%), by volunteer's GP (26/60; 43%). Background of PI influenced consent, reporting and disclosure practice.
Conclusion
There is wide variation in handling IFs in UK imaging research. Much of the current practice contravenes the vague existing legal and ethical guidelines, and is unlikely to be in the best interests of volunteers or researchers.
The term “incidental finding” may give the impression that an unexpected finding is trivial, but an incidental finding (IF) can be life-changing [1]. One definition is “a finding that has potential health or reproductive importance which is discovered in the course of conducting research, but is beyond the aims of the study” [2]. The wider use of imaging in research is making incidental imaging findings more common, and recent academic and popular press editorials have highlighted the need for better management [3,4]. Advancing research practice is controversial and has led to recent debate [5] and deadlock [6] among imaging researchers in Europe and North America.
IFs are common in research imaging. Their prevalence on brain MRI was 2.7% across 16 studies involving 19 559 participants [7] and may be as high as 12.8% on body MRI [8]. Extracolonic IFs at CT colonography require further investigation, medical or surgical intervention in 5–8% [9]. There are, therefore, important implications for informed consent, clinical review of images and mechanisms for notifying the subject. For the volunteers themselves, an IF may have serious implications for health, employment, medical or life insurance, and their state of mind. Furthermore, irrelevant imaging features incorrectly identified as pathological by non-radiologically trained researchers may cause unnecessary distress.
We recently reviewed UK, European and other international legal and ethical guidance on management of IFs, and limited available information on volunteers' expectations. Current recommendations are consistent with the principles that research volunteers should be informed of how their research images will be managed, that there should be measures for identifying and acting on IFs, and information should be disclosed to the subject and his or her responsible physician in a timely, sensitive and appropriate manner [10]. However, this guidance is hard to find and ambiguous, and does not distinguish management of imaging from other types of research sample. We suspected that management of IFs varies considerably between imaging research centres, but lacked reliable data on current practice. We have therefore surveyed UK imaging researchers to determine how IFs are currently managed to provide baseline data to inform future UK practice.
Methods
Participants
The UK National Research Ethics Service (NRES) provided written confirmation that ethical approval was not necessary for this questionnaire-based study (Ref: 04/12). Eligible participants were lead or principle investigators (PIs) of at least one imaging research study using “healthy” volunteers between 2006 and 2009 that had been approved by a UK ethics committee. No national database of imaging researchers exists; we therefore identified potential participants' contact details through research institution websites, and by liaison with relevant managers, heads of departments and known researchers.
Survey design
We produced hard-copy and automated internet versions of the questionnaire (SurveyMonkey.com, Portland, OR). We used tailored design and bimodal methodology for internet surveys to optimise quality and participant response [11-14]. All responses were anonymous. The definition of an IF was highlighted at the start of the questionnaire [2]. PIs were asked to answer the questionnaire in relation to their latest imaging study and to confirm that they had ethics approval for that study. We sought information on participant background (medical/non-medical; radiologist/non-radiologist; number of years as PI), type of research (involving children or not), any policy on IFs at their site, information given to volunteers, involvement of radiologists in reporting of scans and mechanisms for disclosure of any IFs (Appendix A). We defined medical as possessing a medical degree (Bachelor of Medicine and Surgery, or equivalent) and currently practising medicine. We sought information both on current practice and on what PIs considered to be ideal practice (defined as “practice without funding or time constraints”).
Statistical analysis
We compiled data from the completed questionnaires in Microsoft Excel (Microsoft Corp., Seattle, WA) and tested for normality (http://home.ubalt.edu/ntsbarsh/Business-stat/otherapplets/Descriptive.htm). We used the two-tailed Yates corrected χ2 test (or for small numbers Fisher's exact test) for comparisons between groups (http://statpages.org/ctab2x2.html), except for non-parametric continuous and ordered categorical data (e.g. size of institution, years as PI, trends in reporting), in which cases we used the two-tailed Mann–Whitney U-test (http://elegans.swmed.edu/∼leon/stats/utest.cgi). Statistical significance was set at p<0.05. The null hypothesis was that there would be no difference between PIs in current UK practice in the management of IFs; we also explored differences in ideal practice.
Results
Subject characteristics
We identified 160 potential participants, although 3 (2%) could not be contacted and 14 (9%) said that they were ineligible. Of the 146 eligible participants, 63 (43%; on an “intention to survey” basis) completed the questionnaire (Table 1). Non-medical PIs were more likely than medical PIs to image the brain (Yates corrected χ2 test p<0.0001) and use functional MRI (p<0.0001), but were no more likely to image children (Fisher's exact test p=0.74). Years as PI (Mann–Whitney U-test p=0.75) or size of institution (p=0.54) did not differ between medical and non-medical PIs.
Table 1. Background respondent characteristics. This table demonstrates the background characteristics of the 63 (43%) principal investigators (PIs) responding to this survey.
| Characteristic | Value n (%) | Characteristic | Value n (%) |
| Most frequent respondents' locations (total n=61) | Most common PI disciplinesc (total n=62) | ||
| London | 16 (26) | Psychologist | 14 (23) |
| Oxford | 11 (18) | Radiologist (not neuroradiologist) | 10 (16) |
| Manchester | 5 (8) | Basic scientist | 9 (15) |
| Neuroradiologist | 8 (13) | ||
| Size of institution based on total number of studentsa (total n=56) | Physicist | 7 (11) | |
| <10000 | 15 (24) | ||
| 10000–19999 | 24 (39) | ||
| 20000–29999 30000–39999 | 8 (13)3 (5) | Most common research modality and methodc,d (total n=61) MRI structural sequences | 45 (74) |
| >40000 | 6 (10) | fMRI | 31 (51) |
| PI type (total n=62) | MRI whole volume brain sequences | 25 (41) | |
| Medicalb Non-medical | 35 (57)27 (44) | MRI diffusion tensor imaging MR spectroscopy | 21 (34)18 (31) |
| MRI perfusion imaging | 15 (25) | ||
| MRI diffusion imaging | 14 (23) | ||
| Time as PI (total n=62) | MRI permeability imaging | 7 (12) | |
| Years (median; range) | 10; 1–39 | ||
| Research subject typec (total n=62) | |||
| Adult | 62 (98) | ||
| Child | 10 (16) | ||
| Most common research organ(s) imagedc (total n=62) | |||
| Brain | 47 (76) | ||
| Cardiac | 7 (11) | ||
| Gastrointestinal tract | 5 (8) | ||
a6 PIs marked “not applicable”.
bBachelor of medicine and surgery (or equivalent) and currently practicing medicine.
cRespondents could indicate more than one field.
dStructural sequences include T1, T2, fluid-attenuated inversion-recovery and T2*.
Site-specific guidelines
61 respondents answered this question, of whom 54 (88.5%) had site-specific guidelines, 47 (77%) giving a brief description. Here, most PIs explained that research ethics committee approval was required. Type of PI (medical and non-medical, p=0.69; radiologist and non-radiologist, p=0.38), subject (children or adults, p=0.31), years as PI (p=0.14) and size of institution (p=0.88) were not associated with whether an institution had guidelines.
Consent
Most PIs currently provide information to volunteers on handling of personal data (51/62; 82%), contingency plans (54/62; 87%) and disclosure to a general practitioner (GP)/treating physician (47/62; 76%) should an IF be found (Questions 1–3 in Table 2). Most PIs do not currently provide other information listed in Table 2 (Questions 4–9), and even fewer PIs thought it ideal to provide volunteers with this information.
Table 2. Information given to the volunteer during the process of obtaining consent. This table demonstrates the principal investigator (PI) responses to the question: “during the process of obtaining informed consent from the volunteer by the researcher, information is provided on which of the following?”.
| Answer option(s) | Number of PIs answering ‘Yes’a (total of n=62) |
|
| Current practice n (%) | Ideal practice n (%) | |
| 1. What happens to the patient's personal data | 51 (82) | 26 (42) |
| 2. Contingency plans should any incidental findings be foundb | 54 (87) | 21 (34) |
| 3. Disclosure of any incidental findings to the volunteer's general practitioner/treating clinician | 47 (76) | 16 (26) |
| 4. Disclosure of any incidental findings to the volunteer themselves by a member of the research team | 24 (39) | 16 (26) |
| 5. Whether any incidental findings are likely to be treatable | 13 (21) | 11 (18) |
| 6. Potential benefits of any incidental findings should they be found (e.g. prophylactic intervention) | 16 (26) | 13 (21) |
| 7. Potential harms of any incidental findings should they be found (e.g. medical insurance being affected) | 19 (31) | 19 (31) |
| 8. The extent (if any) of the investigator's responsibility to provide medical services should any incidental finding be found | 26 (42) | 19 (31) |
| 9. Potential future re-contact in anticipation that the data be re-analysed in the future after this project has finished | 23 (37) | 20 (32) |
aPIs were informed that they could choose more than one option.
bExamples are: (a) a suitable mechanism is always in place for disclosure of an incidental finding to the volunteer; (b) the plan is to always not disclose.
Medical PIs were more likely than non-medical PIs to indicate that IFs would be disclosed to the volunteer by a member of the research team (p<0.0001), to indicate whether any IFs were likely to be treatable (p=0.047) and to indicate whether there would be potential future re-contact if the data were to be re-analysed (p=0.017). Radiologists were more likely than non-radiologists to discuss potential benefits of identifying any incidental lesion (p=0.048). The number of years as PI and type of research subjects was not associated with this aspect of practice.
The only difference in ideal practice was that medical PIs were more likely than non-medical PIs to opt for discussion with volunteers regarding the investigator's responsibility to provide medical services should any IF be found (p=0.036).
Radiology reporting
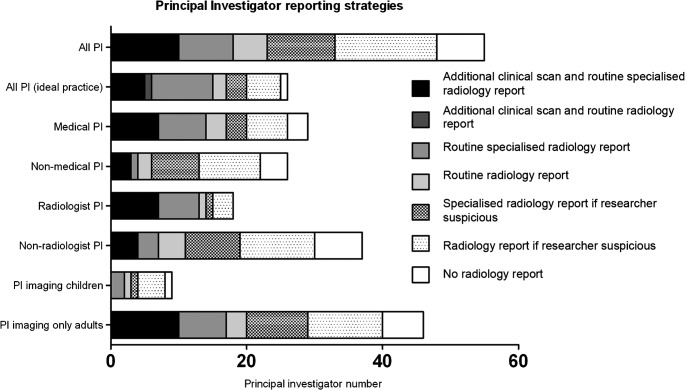
57 (90%) and 28 (44%) of 63 respondents answered the question on radiologist involvement in research imaging in current and ideal practice, respectively (Figure 1). Strategies for image review varied; the most common current policy (15/57; 26%) was to request radiologists to report research scans only when a researcher was suspicious of an IF, although only 5/28 (16%) considered this ideal practice. The commonest ideal reporting strategy was for routine reporting by specialist radiologists 9/28 (29%), but this was current practice for only 8/57 (14%) PIs.
Figure 1.
Research image reporting strategy. Bar charts demonstrating principal investigator (PI) reporting strategies (current practice unless shown otherwise). The degree of shading reflects how proactive a reporting strategy is (darker indicates more proactive).
Medically trained PIs were more likely than non-medical PIs to take a proactive approach (i.e. radiologist reporting of all scans, obtaining additional scans routinely, specialist reporting or combinations thereof) to identify IFs (p=0.041). The tendency towards proactive image review and specialist reporting was even more marked when radiologist PIs were compared with non-radiologists (p=0.007). However, no other PI or subject characteristics were associated with research scan reporting strategy or trend in current or ideal practice.
Disclosure of findings
Of the 56 respondents who answered whether their institution had a standardised contingency plan for disclosure of IFs, 49 (87.5%) confirmed such an arrangement. 35 (62.5%) gave a brief description; in most cases the GP would be informed of any IF. All (26/26) respondents felt that standardised contingency plans were ideal practice.
We received a variety of responses to when, how and by whom IFs were disclosed to volunteers (Table 3). PIs most commonly disclosed IFs to volunteers when judged relevant (27/58, 47%), and a similar percentage thought this ideal (12/26, 46%). Face-to-face disclosure was the most common method of communication (22/54, 41%), and was thought by many to be ideal (19/27, 70%). Disclosure was most commonly by the volunteer's GP (26/60, 43%), which was also thought ideal practice (9/28, 32%).
Table 3. Disclosure of incidental findings to volunteers. This table demonstrates the principal investigator (PI) responses to questions on disclosure of incidental findings to volunteers: “when, if at all, does disclosure take place?” (3a); “how does disclosure take place?” (3b); “who discloses?” (3c); and “if there is no disclosure, why not?” (3d).
| 3a When: frequency of incidental finding disclosure to the volunteer | ||
| Number of PIs answering “yes” |
||
| Current practice n (%) (from a total of n=58)a | Ideal practice n (%) (from a total of n=26) | |
| 1. Incidental findings are never disclosed to the volunteer | 11 (19 ) | 5 (19) |
| 2. Incidental findings are routinely disclosed to the volunteer | 15 (26) | 7 (27) |
| 3. Incidental findings are disclosed to the volunteer if felt to be of relevanceb | 27 (47) | 12 (46) |
| 4. Otherc | 5 (9) | 2 (8) |
| 3b How: method for disclosing an incidental finding to the volunteer | ||
| Number of PIs answering “yes” |
||
| Current practice n (%) (from a total of n=54)a | Ideal practice n (%) (from a total of n=27) | |
| 1. Face-to-face | 22 (41) | 19 (70) |
| 2. By letter | 3 (6) | 0 (0) |
| 3. By e-mail | 0 (0) | 0 (0) |
| 4. By telephone | 3 (6) | 0 (0) |
| 5. Variable and dependent on relevance of incidental findingd | 15 (28) | 6 (22) |
| 6. Othere | 11 (20) | 2 (7) |
| 3c Who: Person disclosing an incidental finding to the volunteer | ||
| Number of PIs answering “yes” |
||
| Current practice n (%) (from a total of n=60)a | Ideal practice n (%) (from a total of n=28)a | |
| 1. By volunteer's own general practitioner | 26 (43) | 9 (32) |
| 2. By a research clinician who is not a member of the research team | 3 (5) | 4 (14) |
| 3. By research team physician | 19 (32) | 6 (21) |
| 4. By research team non-medical member | 0 (0) | 0 (0) |
| 5. By research team radiologist | 2 (3) | 2 (7) |
| 6. Variable and dependent on relevance of incidental findingf | 8 (13) | 7 (25) |
| 7. Otherg | 2 (3) | 0 (0) |
| 3d Why not: reasons for never disclosing incidental findings to research volunteers | ||
| Number of PIs answering “yes”h |
||
| Current practice n (%) (from a total of n=12) | Ideal practice n (%) (from a total of n=12) | |
| 1. Logistical – Too much time and organisation required | 1 (8) | 1 (8) |
| 2. Financial – No funding to support this | 0 (0) | 1 (8) |
| 3. Harmful – Stress for research participants | 5 (42) | 2 (17) |
| 4. Harmful – Medical insurance implications | 1 (8) | 1 (8) |
| 5. Futile – Incidental findings are clinically irrelevant | 1 (8) | 1 (8) |
| 6. Otheri | 7 (58) | 1 (8) |
aTable 3 denominators differ representing the different number of PIs answering individual questions. Some PIs chose two responses when answering questions that specifically asked for a single response. Rather than exclude this data, these responses have been weighted as value 0.5 for each response. Summed values to nearest integer.
bHere, PIs gave examples of “relevance”. An example of a typical response was: “something which may have clinical implications e.g. ovarian cysts or fibroids”.
cHere, PIs described what they meant by “other”. Several explained that rather than deciding themselves whether to disclose an incidental finding or not, it was the GP or medical practitioner who made that decision.
dPIs described this. Most explained that the method of communication was dependent on the GP‘s clinical judgement.
eHere, PIs described what they meant by “other”. All respondents said that the method of communication was dependent on the GP‘s clinical judgement.
fHere, PIs described what they meant by “variable”. Most explained that the person disclosing the incidental finding varies depending on the circumstance. Therefore, it may be appropriate for a radiologist, physician, GP, treating physician or a nurse to disclose, depending on the situation.
gHere, it was unknown who would disclose to the volunteer as historically no incidental findings had been discovered.
hRespondents could select more than one item.
iPIs described what they meant by “other”. Most explained that non-clinical staff reviewed scans therefore disclosure was inappropriate.
Non-medical PIs were significantly more likely never to disclose IFs to the volunteer (p=0.003). The two common reasons given were that non-clinicians process the images (6/12), and that disclosure was harmful and caused stress to the volunteer (5/12). No difference in ideal practice was found between medical and non-medical PIs.
Those who had been PIs for a shorter period of time were more likely to disclose IFs routinely to the volunteer than those who had been PIs for a longer period (p=0.042), but more experienced investigators were more likely than less experienced researchers to disclose IFs to the volunteer if felt to be relevant (p=0.020). There were no other differences in either ideal or current practice by PI type or research subject characteristics.
Medical PIs were more likely to use a research team physician than non-medical researchers, as were radiologists compared with non-radiologists (both p<0.001). Non-medical researchers were more likely to use the volunteer's own GP in current practice (p<0.001) and also felt this was ideal practice (p=0.033).
Discussion
Summary of findings
This survey captured information from almost half of all UK PIs, using healthy volunteers in research imaging, whom we were able to contact through postal and e-mail approaches. Although nearly 90% of respondents had site-specific guidelines for handling IFs in healthy volunteers, we found that this guidance, and the way it is applied, varies widely between sites. Specifically, we demonstrated considerable variation in current practice with regard to (a) information given to volunteers during consent, (b) research scan reporting strategies and (c) the mechanisms of disclosing IFs. Differences in consent, reporting and disclosure practice were associated with investigator background – notably, whether they were medically trained or radiologists, and their level of experience. We additionally obtained views on ideal practice, which we defined as “practice without funding or time constraints”, which also varied among researchers and almost always differed from current practice.
Strengths and weaknesses of the study
Inclusion of only PIs of recent imaging studies, the mixed background characteristics of investigators and the high response rate suggest that the results represent a reasonable cross-section of current UK practice. Our questionnaire was comprehensive and likely to capture the key details of how IFs are currently handled. Nonetheless, this study has limitations. The database used for recruitment was inevitably incomplete, and respondents may represent a biased subgroup. Approximately half the respondents gave no opinion as to what they considered to be ideal practice, reducing the sample size for this aspect of the study; the reasons for poor response in this part of the survey are uncertain. Moreover, although the questionnaire was designed with care, responses may reflect varying interpretation of the questions.
Comparison with studies worldwide
There is limited published data on practice in handling incidental imaging findings in healthy volunteers; all such data are from outside the UK [9,15,16], and the primary aims of those studies differed from our survey. Wider interpretation of previous studies is also limited by low response rate (11%) [16], small sample (12 researchers) [9] or their scope, such as analysis of information from research websites only [15]. Some comparisons can, however, be made with these studies performed outside the UK:
There is wide variability in reporting strategies, with routine radiologist involvement in less than half of all practices, in accordance with the findings of Illes et al (43% in our survey compared with 33% in their United States-based study) [16].
We showed that 12.5% of UK PIs do not have standardised contingency plans for disclosure of IFs. This is lower than the 47% found among neuroimaging investigators in the United States [16] and the 80% in a more general survey of Canadian research institution activity, which also included handling of non-imaging research data [17].
With regard to disclosure, the commonest approach was to disclose relevant IFs in contrast to Siddiki et al, who found the most frequent strategy was routine disclosure (47% in our study compared with Siddiki et als' 42% for relevant reporting; 26% in our study compared with Siddiki et als' 58% for routine reporting) [9]. 19% of PIs in our study never disclosed IFs, compared with <11% in a United States study that also incorporated genomic research [15].
37% of PIs warned volunteers that re-analysis of the data in the future may lead to re-contact after the study has finished. This compares with only 4% in the United States combined imaging and genomic study [15].
Study explanations and relevance from a national and international perspective
Consent
Information provided during consent is inadequate in a notable minority of practice. Although this compares favourably with available data on United States practice, the lack of clear contingency arrangements in over 10% of our sample falls short of mandatory UK NRES requirements that all volunteers are adequately informed about contingency plans, potential benefits and harms should any IFs be found [18]. Not informing the volunteer about who is handling their personal data and for what purposes also contravenes the UK Data Protection Act [19]. Our finding that radiologists were more likely than non-radiologists to discuss potential implications of IFs is likely to reflect greater familiarity with common IFs and the issues that they raise.
Only about 20% of PIs discussed whether any IFs are likely to be treatable, which is a European “Additional Protocol” requirement [20]. This practice was, not surprisingly, more common among medical PIs than non-medical researchers.
WHO/UNESCO also recommend discussion prior to consent of “the extent of the investigator's responsibility to provide medical services to the participant” should an IF be discovered [21]. This ethical guideline is followed by 42% of PIs in current UK practice; here, medical PIs were more likely than non-medical PIs to advocate this as ideal practice.
Just over one-third of PIs warned volunteers that re-analysis of the data in the future may lead to re-contact after the study has finished. This compares to only 4% in the United States [15], although routinely giving such information is recommended there [2]. The higher percentage in the UK may reflect the legal obligation under the Data Protection Act [19], which underpins the NRES instruction [18]. Medical PIs were more likely than non-medical PIs to discuss re-contact, which may again reflect differences in perception of wider implications and limits of researchers' responsibilities.
Reporting
Our findings on radiological reporting suggest wide variation in practice, which ranged from no radiological review of images, through reactive policies (where radiological advice was sought only if an abnormality was noticed by a researcher or radiographer) through to proactive (all images reviewed by a radiologist) and very proactive (where additional imaging was performed routinely to better identify and characterise any incidental abnormalities) reporting [10]. Medical PIs, especially radiologists, tended towards more proactive reporting strategies compared with non-medical investigators.
The only specific UK guidelines on this subject were Medical Devices Agency recommendations that research MRIs are routinely reported by a radiologist [22]. These have recently been superseded by more vague guidelines from the UK Medicines and Healthcare products Regulatory Agency (MHRA) [23].
The reasons for investigators not undertaking routine radiological reporting may reflect lack of access to radiological expertise (particularly for those working in centres not affiliated to clinical services), cost limitations, limited awareness of the frequency and implications of IFs, and opinions as to the extent of responsibility of the study investigator with regard to detection of such abnormalities [24]. Furthermore, policies of no reporting or reactive reporting are sometimes justified on the grounds that detection of IFs lies outside the remit of the research study [25]. This may, however, be counter to subject's expectations [26,27]. Routine proactive radiological review of research images is likely to provide more sensitive and specific detection of significant incidental abnormalities [24]. The benefit to the subject will clearly be greatest where detection of pre-symptomatic treatable disease improves outcome [10]. The images acquired for research studies are often not optimal for detection of disease [24], such that a very proactive approach is advocated by some in the United States on ethical grounds [28] or to avoid litigation [29]. To optimise scientific integrity of studies [5] and facilitate appropriate follow-up [30,31], these strategies may additionally benefit from specialist radiologist involvement (e.g. a neuroradiologist reviewing brain MRI). Our data suggest that proactive policies have also been adopted in a number of UK centres. The advantages of proactive reporting must, however, be balanced against arguments that the purpose of imaging in such studies is not to screen for unexpected pathology [25], minimal evidence base demonstrating benefit and cost implications [10,24].
Policies for review of research imaging in this context remains a controversial area (Appendix B) [5], with particular disparities between clinical and non-clinical imaging groups.
Disclosure
There was variation in practice regarding disclosure of IFs. In the UK, unless the volunteer requests it [19], there is no obligation for IFs to be disclosed [10]. The UK MHRA and WHO/UNESCO recommend that IFs are routinely disclosed during MRI [23] and all [21] research, respectively. Although the adverse effects of disclosure include anxiety, which may be considered unnecessary if the IF is subsequently shown to be of no clinical importance [2,32], available evidence suggests that volunteers always want to be informed of such findings [26,27].
More experienced researchers tended to base decisions to disclose on whether findings were relevant. Disclosure of relevant IFs is concordant with the European Additional Protocol [20]. Only a small percentage of unexpected findings are clinically relevant [33], and some investigators consider it unwise to communicate any except those that are most certain and clinically important [2,6,34].
There are a number of possible reasons for the differences in disclosure policies between medical and non-medical researchers. Non-medical investigators are likely to be less informed about the significance of a finding. Moreover, non-medical investigators' experience will be primarily of a researcher–subject relationship, separate from wider health concerns, whereas medical investigators are familiar with an increasingly non-paternalistic model of the doctor–patient relationship [35], in which sharing of information with the patient is a central doctrine.
Only clinical professionals disclosed IFs to volunteers, consistent with the view that any disclosure to a volunteer is best done by a medical practitioner experienced in communicating sensitive medical information [10]. This goes beyond the European Additional Protocol statement that relevant IFs should be disclosed within a framework of healthcare or counselling, with an appropriate clinical professional supervising the research, although not explicitly disclosing any results themselves [20].
Disclosure was slightly more commonly performed by GPs (43%) than by research team physicians (32%). This accorded broadly with ideal practice, but differs from wishes expressed by United States volunteers in which there was a strong preference for research team (59%) compared to the primary care physician (6%) [27]. The differences may arise in part from the different healthcare models and volunteers' relationships with primary care in the United States. The variability in who discloses IFs in the UK is probably pragmatic, as there is no current guidance. However, implications can be drawn from the UK Department of Health guidance that volunteers' GPs should at least be involved [36,37], thus ensuring effective communication between members of the patient's healthcare team and reliable follow-up [38].
Unanswered questions and future directions
The wide variation in the handling of IFs in the UK is unlikely to be in the best interests of volunteers. Although many issues around consent and disclosure are covered by existing domestic legal and international ethical frameworks, implementation and compliance vary. In the UK and other nations there are no clear national guidelines on imaging review and reporting. Consequently, minimal acceptable standards that accord with informed consent and volunteers' expectations and best interests need to be established. Discrepancies between UK researchers' existing and perceived ideal arrangements may reflect resource constraints, and provide impetus for cost-effective strategies for bridging the gap between current and best practice. Discussion of UK research practice for handling IFs is therefore needed, and will require representation from both clinical and non-clinical researchers, GPs, and patients' representatives. This will allow consensual establishment of practical, lawful and ethically defensible UK national guidelines that can inform NRES advice to ethics committees, and perhaps set a precedent for other nations.
Appendix A
Questionnaire: incidental findings discovered in “healthy” volunteers during research imaging
This questionnaire applies to research involving “healthy” volunteers in all organ systems and using all imaging modalities. This does not apply to “patient” volunteers who are recruited into research projects. Please apply to the last three years only. Please apply to your latest imaging research study that has been approved by an ethics committee.
A. Principal investigator details (please tick all applicable boxes in 1a and 1b)
1a Researcher:
□ Medical researcher – MBBS or equivalent and currently practicing medicine
□ Non-medical researcher
1b Discipline:
□ Basic scientist
□ Computer scientist (informatics)
□ General physician
□ General radiologist
□ Geriatrician
□ Linguist
□ Neurologist
□ Neuroradiologist
□ Neurosurgeon
□ Physicist
□ Psychiatrist
□ Psychologist
□ Stroke physician
□ Other (please describe)
2. Years as principal investigator:
3. Size of institution (student number):
□ Not applicable
□ <10 000
□ 10 000–19 999
□ 20 000–29 999
□ 30 000–39 999
□ >40 000
4. City and county of institution:
5. The research involved (please tick one or more):
□ Adults
□ Children
6. What organ(s) or system(s) were imaged in the project in question? (Please tick one or more):
□ Bone
□ Brain
□ Breast
□ Cardiac
□ Ear, nose throat (please specify below)
□ Endocrine (please specify below)
□ Eye
□ Foetus
□ Female reproductive system
□ Gastrointestional tract
□ Joints
□ Liver and biliary tract
□ Lymphoreticular system
□ Male reproductive system
□ Neuromuscular
□ Renal
□ Respiratory
□ Spinal cord
□ Urinary tract
□ Vascular
□ Other (please describe)
7. What research imaging modalities were used in the project in question? (Please tick one or more)
□ fMRI
□ MRI diffusion tensor imaging
□ MRI perfusion imaging
□ MRI diffusion imaging
□ MRI permeability imaging
□ MRI whole brain volume sequences
□ MRI structural sequences (T1, T2, FLAIR, T2*, etc.)
□ MRI spectroscopy
□ CT perfusion imaging
□ CT structural images
□ Ultrasound
□ SPECT
□ PET
□ Hybrid (please specify)
□ Other (please specify)
The boxes on the left side of the page refer to current practice. The boxes on the right side of the page refer to ideal practice without funding or time constraints. Please tick boxes on both the left and right as appropriate.
B. Informed consent.
During the process of obtaining informed consent of the volunteer by the researcher, information is provided on the following (please tick all applicable boxes)
□ What happens to the patient’s personal data□
□ Contingency plans should any incidental findings be found [examples are: (1) a suitable mechanism is always in place for disclosure of an incidental finding to the volunteer; (2) the plan is to always not disclose]□
□ Disclosure of any incidental findings to the volunteer themselves by a member of the research team□
□ Disclosure of any incidental findings to the volunteer’s general practitioner/treating clinician□
□ Whether any incidental findings are likely to be treatable□
□ Potential benefits of any incidental findings should they be found (e.g. prophylactic intervention) □
□ Potential harms of any incidental findings should they be found (e.g. medical insurance being affected) □
□ The extent (if any) of the investigator’s responsibility to provide medical services should any incidental finding be found□
□ Potential future re-contact in anticipation that the data be re-analysed in the future after this project has finished□
C. Disclosure.
Which one of the following items best describes arrangements for disclosure to the volunteer if an incidental finding is discovered? (Tick once in each column)
□ Incidental findings are never disclosed to the volunteer (if this is the best response please answer all questions except F and G) □
□ Incidental findings are routinely disclosed to the volunteer□
□ Incidental findings are disclosed to the volunteer if felt to be of relevance (please give example of what you consider to be of relevance) □
□ Other (please describe) □
D. Non-disclosure.
Only answer this question if you chose response 1 in question C. Which of the following item(s) describes why incidental findings are never disclosed to research volunteers? (Please select one or more item)
□ Logistical – too much time and organisation required□
□ Financial – no funding to support this□
□ Harmful – stress for research participant□
□ Harmful – medical insurance implications□
□ Futile – incidental findings are clinically irrelevant□
□ Other (please describe)
E. Standardised contingency plan.
Is there a standardised contingency plan for incidental findings? [Examples are: (1) a suitable mechanism is always in place for disclosure of an incidental finding to the volunteer; (2) the plan is always not to disclose]
□ Yes□
□ No□
If yes, please describe briefly:
F. Disclosure method.
Which of the following stems best describes how incidental findings are disclosed to the volunteer [please tick a single box in each column in F(a) and F(b)]
F(a) By whom:
□ By volunteer’s own general practitioner□
□ By a clinician who is not member of research team□
□ By research team physician□
□ By research team non-medical member□
□ By research team radiologist□
□ Variable and dependent on relevance of incidental finding (please describe) □
□ Other (please describe) □
F(b) Means of communication:
□ Face to face□
□ By letter□
□ By e-mail □
□ By telephone□
□ Variable and dependent on relevance of incidental finding (please describe) □
□ Other (please describe) □
G. Radiology reporting.
Which one of the following stems best describes how radiologists report research scans?
□ Radiologists do not report research scans□
□ Radiologists report research scans only if a researcher is suspicious of an incidental finding□
□ Radiologists report research scans in their specialist field (e.g. neuroradiologists reporting brains) only if a researcher is suspicious of an incidental finding□
□ Radiologists report all research scans routinely□
□ Radiologists report all research scans routinely in their specialist field (e.g. neuroradiologists reporting brains) □
□ Research scans and also clinical scans additional to those necessary for the project are routinely obtained and all are reported by a radiologist□
□ Research scans and also clinical scans additional to those necessary for the project are routinely obtained and all are reported by a radiologist in their specialist field (e.g. neuroradiologists reporting brains) □
□ Other (please describe) □
Appendix B
A selection of comments invited following survey completion.
I do not believe that it is cost effective or practical to require additional “clinical” scans and radiological reading on all studies. I believe our current practice of following up only on suspicious findings, and clearly badging the imaging we do as “research that may have no diagnostic value”, is the right one.
If scans are routinely reported it would add significantly to the costs of research, for no obvious gain.
The reporting is time-consuming and not trivial, but having run a research scanner for twelve years and research imaging on (UK) National Health Service scanners before that, we have decided that there isn’t any other ethical way of doing it. It’s not fair on the volunteer not to look after their scan findings.
Long overdue. We must implement a standardised best practice across all institutions which have imaging equipment capable of detecting pathology. Too many “toys” are being installed in basic science/physics/psychology departments without a clear understanding of the ethical implications.
References
- 1.Anonymous How volunteering for an MRI scan changed my life. Nature 2005;434:7029. [DOI] [PubMed] [Google Scholar]
- 2.Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Wright-Clayton E, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics 2008;36:219–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staff Editorial Dilemmas posed by chance research results: what should researchers do when they discover unlooked-for information that has serious implications for a subject's health? The answer is far from straightforward. New Scientist 2008;199:5 [Google Scholar]
- 4.Woodward CI, Toms AP. Incidental findings in “normal” volunteers. Clin Radiol 2009;64:951–3 [DOI] [PubMed] [Google Scholar]
- 5.Hentschel F, von Kummer R. The response of the German Society of Neuroradiology to the guideline “ethically appropriate reaction to incidental imaging findings in brain research”. Clin Neuroradiol 2009;19:108–10 [DOI] [PubMed] [Google Scholar]
- 6.Illes J, Kirschen MP, Edwards E, Stanford LR, Bandettini P, Cho MK, et al. Incidental findings in brain imaging research. Science 2006;311:783–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris Z, Whiteley WN, Longstreth JWT, Weber F, Lee YC, Tsushima Y, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2009;339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin S, Cobbold J, Lim A, Eliahoo J, Thomas EL, Mehta SR, et al. Incidental findings in healthy control research subjects using whole-body MRI. Eur J Radiol 2009;72:529–33 [DOI] [PubMed] [Google Scholar]
- 9.Siddiki H, Fletcher JG, McFarland B, Dajani N, Orme N, Koenig B, et al. Incidental findings in CT colonography: literature review and survey of current practice. J Law Med Ethics 2008;36:320–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth TC, Jackson A, Wardlaw JM, Taylor SA, Waldman AD. Incidental findings found in “healthy” volunteers during imaging performed for research: current legal and ethical implications. Br J Radiol 2010;83:456–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillman DA. Mail and internet surveys: the tailored design method. New York, NY: John Wiley and Sons Inc., 2000 [Google Scholar]
- 12.Brashears T. Low expense, high return: a bimodal methodology for internet survey implementation. 2003. Available from: http://www.depts.ttu.edu/aged/research/brashaerssurveyabstract [accessed 2008 May 29]
- 13.Rosenbaum J, Lidz CW. Maximizing the results of internet surveys. Available from: http://www.unassmed.edu/uploadedFiles/Brief28Surveys 4. 2007. 29 [accessed 2008 May 29]
- 14.Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Methods to increase response rates to postal questionnaires. Cochrane Database Syst Rev 2009, Issue 3 Art.No.:MR000008. DOI: 10.1002/14651858.MR000008. pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrenz F, Sobotka S. Empirical analysis of current approaches to incidental findings. J Law Med Ethics 2008;36:249–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illes J, Kirschen MP, Karetsky K, Kelly M, Saha A, Desmond JE, et al. Discovery and disclosure of incidental findings in neuroimaging research. J Magn Reson Imaging 2004;20:743–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macneil SD, Fernandez CV. Attitudes of research ethics board chairs towards disclosure of research results to participants: results of a national survey. J Med Ethics 2007;33:549–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Research Ethics Service. National Patient Safety Agency information sheets and consent forms. Guidance for researchers and reviewers. 2007. Available from: http://www.nres.npsa.nhs.uk/EasySiteWeb/GatewayLink.aspx?alld=4757 [accessed 2009 April 24]
- 19.HM Government. The Data Protection Act 1998. Available from: http://www.opsi.gov.uk [accessed 2008 June 10]
- 20.Council of Europe Steering Committee on Bioethics. Additional protocol to the convention on human rights and biomedicine concerning biomedical research. 2004. Available from: http://conventions.coe.int [accessed 2008 May 8]
- 21.The Council for International Organizations of Medical Sciences (CIOMS) International ethical guidelines for biomedical research involving human subjects. 2002. Available from: http://www.cioms.ch [accessed 2008 May 8]
- 22.Department of Health UK, Medical Devices Agency. Guidelines for magnetic resonance equipment in clinical use. 2002. Available from: http://www.mhra.gov.uk/home/idcplg?IdcService [accessed 2008 August 6]
- 23.Department of Health UK. Devices bulletin. Safety guidelines for Magnetic Resonance Equipment in Clinical Use. 2007. Available from: http://www.mhra.gov.uk/home/idcplg?IdcService [accessed 2010 May 1]
- 24.Royal JM, Peterson BS. The risks and benefits of searching for incidental findings in MRI research scans. J Law Med Ethics 2008;36:305–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller FG, Mello MM, Joffe S. Incidental findings in human research: What do investigators owe research participants? J Law Med Ethics 2008;36:271–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellcome Trust Clinical Research Facility. How should we manage incidental abnormalities found on research scans? 2009. Available from: http://www.wtcrf.ed.ac.uk/education/Seminar%20Archive.htm [accessed 2009 August 1]
- 27.Kirschen MP, Jaworska A, Illes J. Subjects' expectations in neuroimaging research. J Magn Reson Imaging 2006;23:205–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson HS. Incidental findings and ancillary-care obligations. J Law Med Ethics 2008;36:256–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milstein AC. Research malpractice and the issue of incidental findings. J Law Med Ethics 2008;36:356–60 [DOI] [PubMed] [Google Scholar]
- 30.Grainger R, Stuckey S, O'Sullivan R, Davis SR, Ebeling PR, Wluka AE. What is the clinical and ethical importance of incidental abnormalities found by knee MRI? Arthritis Res Ther 2008;10:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim BS, Illes J, Kaplan RT, Reiss A, Atlas SW. Incidental findings on pediatric MR images of the brain. AJNR Am J Neuroradiol 2002;23:1674–7 [PMC free article] [PubMed] [Google Scholar]
- 32.Kumra S, Ashtari M, Anderson B, Cervellione KL, Kan L. Ethical and practical considerations in the management of incidental findings in pediatric MRI studies. J Am Acad Child Adolesc Psychiatry 2006;5:1000–6 [DOI] [PubMed] [Google Scholar]
- 33.Illes J, Desmond JE, Huang LF, Raffin TA, Atlas SW. Ethical and practical considerations in managing incidental findings in functional imaging. Brain Cogn 2002;50:358–65 [DOI] [PubMed] [Google Scholar]
- 34.Wilfond BS, Carpenter KJ. Incidental findings in pediatric research. J Law Med Ethics 2008;36:332–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coulter A. Paternalism or partnership? Patients have grown up – and there's no going back. BMJ 1999;319:719–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Department of Health. Governance arrangements for NHS research ethics committees. 2001. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance [accessed 2008 May 8]
- 37.English V, Romano-Critchley G, Sheather J, Sommerville A. Medical ethics today: the BMA's handbook of ethics and law. London, UK: BMJ Books, 2007: 489–534 [Google Scholar]
- 38.Casola G. Whole-body CT scanning: self referral. Fourth International Symposium: Virtual Colonoscopy; 2003 Oct 13–15; Boston, MA: Boston University Medical Center, 2003 [Google Scholar]